Regulation of NT-PGC-1alpha subcellular localization and function by protein kinase A-dependent modulation of nuclear export by CRM1
- PMID: 20351112
- PMCID: PMC2878565
- DOI: 10.1074/jbc.M109.083121
Regulation of NT-PGC-1alpha subcellular localization and function by protein kinase A-dependent modulation of nuclear export by CRM1
Abstract
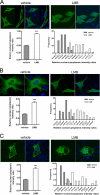
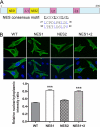
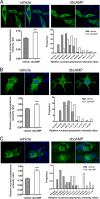
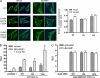
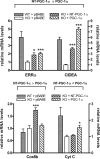
Peroxisome proliferator-activated receptor gamma co-activator-1alpha (PGC-1alpha) plays a central role in the regulation of cellular energy metabolism and metabolic adaptation to environmental and nutritional stimuli. We recently described a novel, biologically active splice variant of PGC-1alpha (NT-PGC-1alpha, amino acids 1-270) that retains the ability to interact with and transactivate nuclear hormone receptors through its N-terminal transactivation domain. Whereas PGC-1alpha is an unstable nuclear protein sensitive to ubiquitin-mediated targeting to the proteasome, NT-PGC-1alpha is relatively stable and predominantly cytoplasmic, suggesting that its ability to interact with and activate nuclear receptors and transcription factors is dependent upon regulated access to the nucleus. We provide evidence that NT-PGC-1alpha interacts with the nuclear exportin, CRM1, through a specific leucine-rich domain (nuclear export sequence) that regulates its export to the cytoplasm. The nuclear export of NT-PGC-1alpha is inhibited by protein kinase A-dependent phosphorylation of Ser-194, Ser-241, and Thr-256 on NT-PGC-1alpha, which effectively increases its nuclear concentration. Using site-directed mutagenesis to prevent or mimic phosphorylation at these sites, we show that the transcriptional activity of NT-PGC-1alpha is regulated in part through regulation of its subcellular localization. These findings suggest that the function of NT-PGC-1alpha as a transcriptional co-activator is regulated by protein kinase A-dependent inhibition of CRM1-mediated export from the nucleus.
Figures
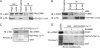


References
-
- Handschin C., Spiegelman B. M. (2006) Endocr. Rev. 27, 728–735 - PubMed
-
- Puigserver P., Wu Z., Park C. W., Graves R., Wright M., Spiegelman B. M. (1998) Cell 92, 829–839 - PubMed
-
- Wu Z., Puigserver P., Andersson U., Zhang C., Adelmant G., Mootha V., Troy A., Cinti S., Lowell B., Scarpulla R. C., Spiegelman B. M. (1999) Cell 98, 115–124 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

