Cooperation between multiple microbial pattern recognition systems is important for host protection against the intracellular pathogen Legionella pneumophila
- PMID: 20351139
- PMCID: PMC2876572
- DOI: 10.1128/IAI.00243-10
Cooperation between multiple microbial pattern recognition systems is important for host protection against the intracellular pathogen Legionella pneumophila
Abstract
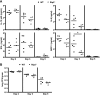
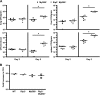
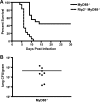
Multiple pattern recognition systems have been shown to initiate innate immune responses to microbial pathogens. The degree to which these detection systems cooperate with each other to provide host protection is unknown. Here, we investigated the importance of several immune surveillance pathways in protecting mice against lethal infection by the intracellular pathogen Legionella pneumophila, the causative agent of a severe pneumonia called Legionnaires' disease. Rip2 and Naip5/NLRC4 signaling was found to contribute to the innate immune response generated against L. pneumophila in the lung. Elimination of Rip2 or Naip5/NLRC4 signaling in MyD88-deficient mice resulted in increased replication and dissemination of L. pneumophila and higher rates of mortality. Irradiated wild-type mice receiving bone marrow cells from pattern recognition receptor-deficient mice displayed L. pneumophila infection phenotypes similar to those of donor mice. Rip2 and Naip5/NLRC4 signaling provided additive effects in protecting MyD88-deficient mice from lethal infection by L. pneumophila, with the contribution of Naip5/NLRC4 being slightly greater than that of Rip2. Thus, activation of the Rip2, MyD88, and Naip5/NLRC4 signaling pathways triggers a coordinated and synergistic response that protects the host against lethal infection by L. pneumophila. These data provide new insight into how different pattern recognition systems interact functionally to generate innate immune responses that protect the host from lethal infection by activating cellular pathways that restrict intracellular replication of L. pneumophila and by recruiting to the site of infection additional phagocytes that eliminate extracellular bacteria.
Figures



References
-
- Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143-150. - PubMed
-
- Akhter, A., M. A. Gavrilin, L. Frantz, S. Washington, C. Ditty, D. Limoli, C. Day, A. Sarkar, C. Newland, J. Butchar, C. B. Marsh, M. D. Wewers, S. Tridandapani, T. D. Kanneganti, and A. O. Amer. 2009. Caspase-7 activation by the Nlrc4/Ipaf inflammasome restricts Legionella pneumophila infection. PLoS Pathog. 5:e1000361. - PMC - PubMed
-
- Amer, A., L. Franchi, T. D. Kanneganti, M. Body-Malapel, N. Ozoren, G. Brady, S. Meshinchi, R. Jagirdar, A. Gewirtz, S. Akira, and G. Nunez. 2006. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J. Biol. Chem. 281:35217-35223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

