Specificity and stoichiometry of subunit interactions in the human telomerase holoenzyme assembled in vivo
- PMID: 20351177
- PMCID: PMC2876521
- DOI: 10.1128/MCB.00151-10
Specificity and stoichiometry of subunit interactions in the human telomerase holoenzyme assembled in vivo
Abstract
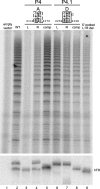
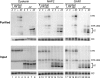
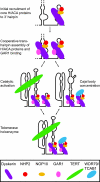
The H/ACA motif of human telomerase RNA (hTR) directs specific pathways of endogenous telomerase holoenzyme assembly, function, and regulation. Similarities between hTR and other H/ACA RNAs have been established, but differences have not been explored even though unique features of hTR H/ACA RNP assembly give rise to telomerase deficiency in human disease. Here, we define hTR H/ACA RNA and RNP architecture using RNA accumulation, RNP affinity purification, and primer extension activity assays. First, we evaluate alternative folding models for the hTR H/ACA motif 5' hairpin. Second, we demonstrate an unanticipated and surprisingly general asymmetry of 5' and 3' hairpin requirements for H/ACA RNA accumulation. Third, we establish that hTR assembles not one but two sets of all four of the H/ACA RNP core proteins, dyskerin, NOP10, NHP2, and GAR1. Fourth, we address a difference in predicted specificities of hTR association with the holoenzyme subunit WDR79/TCAB1. Together, these results complete the analysis of hTR elements required for active RNP biogenesis and define the interaction specificities and stoichiometries of all functionally essential human telomerase holoenzyme subunits. This study uncovers unexpected similarities but also differences between telomerase and other H/ACA RNPs that allow a unique specificity of telomerase biogenesis and regulation.
Figures




References
-
- Bryan, T., L. Marusic, S. Bacchetti, M. Namba, and R. R. Reddel. 1997. The telomere lengthening mechanism in telomerase-negative immortal human cells does not involve the telomerase RNA subunit. Hum. Mol. Genet. 6:921-926. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
