Xenoestrogen-induced regulation of EZH2 and histone methylation via estrogen receptor signaling to PI3K/AKT
- PMID: 20351197
- PMCID: PMC2870935
- DOI: 10.1210/me.2009-0438
Xenoestrogen-induced regulation of EZH2 and histone methylation via estrogen receptor signaling to PI3K/AKT
Abstract
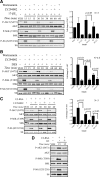
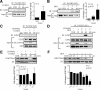
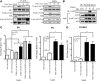
Although rapid, membrane-activated estrogen receptor (ER) signaling is no longer controversial, the biological function of this nongenomic signaling is not fully characterized. We found that rapid signaling from membrane-associated ER regulates the histone methyltransferase enhancer of Zeste homolog 2 (EZH2). In response to both 17beta-estradiol (E2) and the xenoestrogen diethylstilbestrol, ER signaling via phosphatidylinositol 3-kinase/protein kinase B phosphorylates EZH2 at S21, reducing levels of trimethylation of lysine 27 on histone H3 in hormone-responsive cells. During windows of uterine development that are susceptible to developmental reprogramming, activation of this ER signaling pathway by diethylstilbestrol resulted in phosphorylation of EZH2 and reduced levels of trimethylation of lysine 27 on histone H3 in chromatin of the developing uterus. Furthermore, activation of nongenomic signaling reprogrammed the expression profile of estrogen-responsive genes in uterine myometrial cells, suggesting this as a potential mechanism for developmental reprogramming caused by early-life exposure to xenoestrogens. These data demonstrate that rapid ER signaling provides a direct linkage between xenoestrogen-induced nuclear hormone receptor signaling and modulation of the epigenetic machinery during tissue development.
Figures



Similar articles
-
Environmental estrogens differentially engage the histone methyltransferase EZH2 to increase risk of uterine tumorigenesis.Mol Cancer Res. 2012 Apr;10(4):546-57. doi: 10.1158/1541-7786.MCR-11-0605. Mol Cancer Res. 2012. PMID: 22504913 Free PMC article.
-
Histone methyltransferase EZH2 is transcriptionally induced by estradiol as well as estrogenic endocrine disruptors bisphenol-A and diethylstilbestrol.J Mol Biol. 2014 Oct 9;426(20):3426-41. doi: 10.1016/j.jmb.2014.07.025. Epub 2014 Aug 1. J Mol Biol. 2014. PMID: 25088689
-
Overexpression of Enhancer of zeste homolog 2 with trimethylation of lysine 27 on histone H3 in adult T-cell leukemia/lymphoma as a target for epigenetic therapy.Haematologica. 2011 May;96(5):712-9. doi: 10.3324/haematol.2010.028605. Epub 2011 Jan 12. Haematologica. 2011. PMID: 21228036 Free PMC article.
-
Epigenetic regulation of signaling pathways in cancer: role of the histone methyltransferase EZH2.J Gastroenterol Hepatol. 2011 Jan;26(1):19-27. doi: 10.1111/j.1440-1746.2010.06447.x. J Gastroenterol Hepatol. 2011. PMID: 21175789 Review.
-
Epigenomic reprogramming of the developing reproductive tract and disease susceptibility in adulthood.Birth Defects Res A Clin Mol Teratol. 2011 Aug;91(8):666-71. doi: 10.1002/bdra.20827. Epub 2011 Jun 7. Birth Defects Res A Clin Mol Teratol. 2011. PMID: 21656660 Free PMC article. Review.
Cited by
-
Molecular pathways: environmental estrogens activate nongenomic signaling to developmentally reprogram the epigenome.Clin Cancer Res. 2013 Jul 15;19(14):3732-7. doi: 10.1158/1078-0432.CCR-13-0021. Epub 2013 Apr 2. Clin Cancer Res. 2013. PMID: 23549878 Free PMC article.
-
Cooperation of Genomic and Rapid Nongenomic Actions of Estrogens in Synaptic Plasticity.Mol Neurobiol. 2017 Aug;54(6):4113-4126. doi: 10.1007/s12035-016-9979-y. Epub 2016 Jun 20. Mol Neurobiol. 2017. PMID: 27324789 Free PMC article. Review.
-
Reactive oxygen species contribute to arsenic-induced EZH2 phosphorylation in human bronchial epithelial cells and lung cancer cells.Toxicol Appl Pharmacol. 2014 May 1;276(3):165-70. doi: 10.1016/j.taap.2014.02.005. Epub 2014 Feb 25. Toxicol Appl Pharmacol. 2014. PMID: 24582688 Free PMC article.
-
Early Life Adverse Environmental Exposures Increase the Risk of Uterine Fibroid Development: Role of Epigenetic Regulation.Front Pharmacol. 2016 Mar 1;7:40. doi: 10.3389/fphar.2016.00040. eCollection 2016. Front Pharmacol. 2016. PMID: 26973527 Free PMC article. Review.
-
Genetic and epigenetic regulation of AHR gene expression in MCF-7 breast cancer cells: role of the proximal promoter GC-rich region.Biochem Pharmacol. 2012 Sep 1;84(5):722-35. doi: 10.1016/j.bcp.2012.06.013. Epub 2012 Jun 21. Biochem Pharmacol. 2012. PMID: 22728919 Free PMC article.
References
-
- Acconcia F, Kumar R 2006 Signaling regulation of genomic and nongenomic functions of estrogen receptors. Cancer Lett 238:1–14 - PubMed
-
- Gruber CJ, Tschugguel W, Schneeberger C, Huber JC 2002 Production and actions of estrogens. N Engl J Med 346:340–352 - PubMed
-
- Nagel SC, vom Saal FS, Welshons WV 1999 Developmental effects of estrogenic chemicals are predicted by an in vitro assay incorporating modification of cell uptake by serum. J Steroid Biochem Mol Biol 69:343–357 - PubMed

