Compartmentalized MHC class I antigen processing enhances immunosurveillance by circumventing the law of mass action
- PMID: 20351281
- PMCID: PMC2872426
- DOI: 10.1073/pnas.0910997107
Compartmentalized MHC class I antigen processing enhances immunosurveillance by circumventing the law of mass action
Abstract
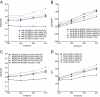
MHC class I molecules function to display peptides generated from cellular and pathogen gene products for immune surveillance by CD8(+) T cells. Cells typically express approximately 100,000 class I molecules, or approximately 1 per 30,000 cellular proteins. Given "one protein, one peptide" representation, immunosurveillance would be heavily biased toward the most abundant cell proteins. Cells use several mechanisms to prevent this, including the predominant use of defective ribosomal products (DRiPs) to generate peptides from nascent proteins and, as we show here, compartmentalization of DRiP peptide generation to prevent competition from abundant cytosolic peptides. This provides an explanation for the exquisite ability of T cells to recognize peptides generated from otherwise undetected gene products.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
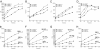

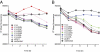

References
-
- Shastri N, Schwab S, Serwold T. Producing nature's gene chips: The generation of peptides for display by MHC class I molecules. Annu Rev Immunol. 2002;20:463–493. - PubMed
-
- Yewdell JW, Reits E, Neefjes J. Making sense of mass destruction: Quantitating MHC class I antigen presentation. Nat Rev Immunol. 2003;3:952–961. - PubMed
-
- Yewdell JW, Nicchitta CV. The DRiP hypothesis decennial: Support, controversy, refinement and extension. Trends Immunol. 2006;27:368–373. - PubMed
-
- Fruci D, et al. Quantifying recruitment of cytosolic peptides for HLA class I presentation: Impact of TAP transport. J Immunol. 2003;170:2977–2984. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

