Identification of neuronal subpopulations that project from hypothalamus to both liver and adipose tissue polysynaptically
- PMID: 20351287
- PMCID: PMC2872469
- DOI: 10.1073/pnas.1002790107
Identification of neuronal subpopulations that project from hypothalamus to both liver and adipose tissue polysynaptically
Abstract
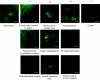
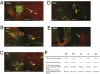
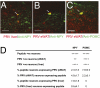
The autonomic nervous system regulates fuel availability and energy storage in the liver, adipose tissue, and other organs; however, the molecular components of this neural circuit are poorly understood. We sought to identify neural populations that project from the CNS indirectly through multisynaptic pathways to liver and epididymal white fat in mice using pseudorabies virus strains expressing different reporters together with BAC transgenesis and immunohistochemistry. Neurons common to both circuits were identified in subpopulations of the paraventricular nucleus of the hypothalamus (PVH) by double labeling with markers expressed in viruses injected in both sites. The lateral hypothalamus and arcuate nucleus of the hypothalamus and brainstem regions (nucleus of the solitary tract and A5 region) also project to both tissues but are labeled at later times. Connections from these same sites to the PVH were evident after direct injection of virus into the PVH, suggesting that these regions lie upstream of the PVH in a common pathway to liver and adipose tissue (two metabolically active organs). These common populations of brainstem and hypothalamic neurons express neuropeptide Y and proopiomelanocortin in the arcuate nucleus, melanin-concentrating hormone, and orexin in the lateral hypothalamus and in the corticotrophin-releasing hormone and oxytocin in the PVH. The delineation of this circuitry will facilitate a functional analysis of the possible role of these potential command-like neurons to modulate autonomic outflow and coordinate metabolic responses in liver and adipose tissue.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Cannon WB. Organization for physiological homeostasis. Physiol Rev. 1929;9:399–431.
-
- Anderson EA, Wallin BG, Mark AL. Dissociation of sympathetic nerve activity in arm and leg muscle during mental stress. Hypertension. 1987;9:III114–III119. - PubMed
-
- Brito MN, Brito NA, Baro DJ, Song CK, Bartness TJ. Differential activation of the sympathetic innervation of adipose tissues by melanocortin receptor stimulation. Endocrinology. 2007;148:5339–5347. - PubMed
-
- Püschel GP. Control of hepatocyte metabolism by sympathetic and parasympathetic hepatic nerves. Anat Rec A Discov Mol Cell Evol Biol. 2004;280:854–867. - PubMed
-
- Fredholm BB, Karlsson J. Metabolic effects of prolonged sympathetic nerve stimulation in canine subcutaneous adipose tissue. Acta Physiol Scand. 1970;80:567–576. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

