Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation
- PMID: 20351312
- PMCID: PMC2890190
- DOI: 10.1182/blood-2009-10-249540
Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation
Abstract
Dendritic cells (DCs) are key regulators of innate and acquired immunity. The maturation of DCs is directed by signal transduction events downstream of toll-like receptors (TLRs) and other pattern recognition receptors. Here, we demonstrate that, in mouse DCs, TLR agonists stimulate a profound metabolic transition to aerobic glycolysis, similar to the Warburg metabolism displayed by cancer cells. This metabolic switch depends on the phosphatidyl inositol 3'-kinase/Akt pathway, is antagonized by the adenosine monophosphate (AMP)-activated protein kinase (AMPK), and is required for DC maturation. The metabolic switch induced by DC activation is antagonized by the antiinflammatory cytokine interleukin-10. Our data pinpoint TLR-mediated metabolic conversion as essential for DC maturation and function and reveal it as a potential target for intervention in the control of excessive inflammation and inappropriately regulated immune responses.
Figures
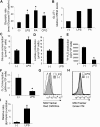
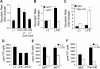
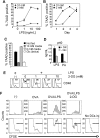
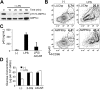
References
-
- Barton GM, Medzhitov R. Control of adaptive immune responses by Toll-like receptors. Curr Opin Immunol. 2002;14(3):380–383. - PubMed
-
- Krawczyk CM, Shen H, Pearce EJ. Functional plasticity in memory T helper cell responses. J Immunol. 2007;178(7):4080–4088. - PubMed
-
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4(7):499–511. - PubMed
-
- Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5(10):987–995. - PubMed
-
- Huang Q, Liu D, Majewski P, et al. The plasticity of dendritic cell responses to pathogens and their components. Science. 2001;294(5543):870–875. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

