Neuropeptide Y directly inhibits neuronal activity in a subpopulation of gonadotropin-releasing hormone-1 neurons via Y1 receptors
- PMID: 20351316
- PMCID: PMC2875836
- DOI: 10.1210/en.2009-1198
Neuropeptide Y directly inhibits neuronal activity in a subpopulation of gonadotropin-releasing hormone-1 neurons via Y1 receptors
Abstract
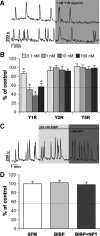
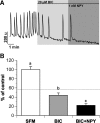
Neuropeptide Y (NPY), a member of the pancreatic polypeptide family, is an orexigenic hormone. GnRH-1 neurons express NPY receptors. This suggests a direct link between metabolic function and reproduction. However, the effect of NPY on GnRH-1 cells has been variable, dependent on metabolic and reproductive status of the animal. This study circumvents these issues by examining the role of NPY on GnRH-1 neuronal activity in an explant model that is based on the extra-central nervous system origin of GnRH-1 neurons. These prenatal GnRH-1 neurons express many receptors found in GnRH-1 neurons in the brain and use similar transduction pathways. In addition, these GnRH-1 cells exhibit spontaneous and ligand-induced oscillations in intracellular calcium as well as pulsatile calcium-controlled GnRH-1 release. Single-cell PCR determined that prenatal GnRH-1 neurons express the G protein-coupled Y1 receptor (Y1R). To address the influence of NPY on GnRH-1 neuronal activity, calcium imaging was used to monitor individual and population dynamics. NPY treatment, mimicked with Y1R agonist, significantly decreased the number of calcium peaks per minute in GnRH-1 neurons and was prevented by a Y1R antagonist. Pertussis toxin blocked the effect of NPY on GnRH-1 neuronal activity, indicating the coupling of Y1R to inhibitory G protein. The NPY-induced inhibition was independent of the adenylate cyclase pathway but mediated by the activation of G protein-coupled inwardly rectifying potassium channels. These results indicate that at an early developmental stage, GnRH-1 neuronal activity can be directly inhibited by NPY via its Y1R.
Figures


Similar articles
-
Neuropeptide Y induces gonadotropin-releasing hormone gene expression directly and through conditioned medium from mHypoE-38 NPY neurons.Regul Pept. 2009 Aug 7;156(1-3):96-103. doi: 10.1016/j.regpep.2009.04.005. Epub 2009 Apr 14. Regul Pept. 2009. PMID: 19371763
-
Involvement of neuropeptide Y Y1 receptors in the regulation of neuroendocrine corticotropin-releasing hormone neuronal activity.Endocrinology. 2007 Aug;148(8):3666-73. doi: 10.1210/en.2006-1730. Epub 2007 Apr 26. Endocrinology. 2007. PMID: 17463058
-
Functional interaction between neuropeptide Y receptors and modulation of calcium channels in the rat hippocampus.Neuropharmacology. 2003 Feb;44(2):282-92. doi: 10.1016/s0028-3908(02)00382-9. Neuropharmacology. 2003. PMID: 12623227
-
Control of GnRH neuronal activity by metabolic factors: the role of leptin and insulin.Mol Cell Endocrinol. 2006 Jul 25;254-255:133-9. doi: 10.1016/j.mce.2006.04.023. Epub 2006 Jun 6. Mol Cell Endocrinol. 2006. PMID: 16757107 Review.
-
The role of Neuropeptide Y in fear conditioning and extinction.Neuropeptides. 2016 Feb;55:111-26. doi: 10.1016/j.npep.2015.09.007. Epub 2015 Sep 25. Neuropeptides. 2016. PMID: 26444585 Review.
Cited by
-
Neuroendocrine pathways mediating nutritional acceleration of puberty: insights from ruminant models.Front Endocrinol (Lausanne). 2011 Dec 27;2:109. doi: 10.3389/fendo.2011.00109. eCollection 2011. Front Endocrinol (Lausanne). 2011. PMID: 22654842 Free PMC article.
-
Investigating GABA Neuron-Specific Androgen Receptor Knockout in two Hyperandrogenic Models of PCOS.Endocrinology. 2024 May 27;165(7):bqae060. doi: 10.1210/endocr/bqae060. Endocrinology. 2024. PMID: 38788194 Free PMC article.
-
BPA Directly Decreases GnRH Neuronal Activity via Noncanonical Pathway.Endocrinology. 2016 May;157(5):1980-90. doi: 10.1210/en.2015-1924. Epub 2016 Mar 2. Endocrinology. 2016. PMID: 26934298 Free PMC article.
-
Genetic effects of polymorphisms in candidate genes and the QTL region on chicken age at first egg.BMC Genet. 2011 Apr 15;12:33. doi: 10.1186/1471-2156-12-33. BMC Genet. 2011. PMID: 21492484 Free PMC article.
-
Long non-coding RNAs could act as vectors for paternal heredity of high fat diet-induced obesity.Oncotarget. 2017 Jul 18;8(29):47876-47889. doi: 10.18632/oncotarget.18138. Oncotarget. 2017. PMID: 28599310 Free PMC article.
References
-
- Levine JE, Pau KY, Ramirez VD, Jackson GL 1982 Simultaneous measurement of luteinizing hormone-releasing hormone and luteinizing hormone release in unanesthetized, ovariectomized sheep. Endocrinology 111:1449–1455 - PubMed
-
- Błogowska A, Krzyzanowska-Swiniarska B, Zieliñska D, Rzepka-Górska I 2006 Body composition and concentrations of leptin, neuropeptide Y, β-endorphin, growth hormone, insulin-like growth factor-I and insulin at menarche in girls with constitutional delay of puberty. Gynecol Endocrinol 22:274–278 - PubMed
-
- Plant TM, Shahab M 2002 Neuroendocrine mechanisms that delay and initiate puberty in higher primates. Physiol Behav 77:717–722 - PubMed
-
- Pralong FP, Voirol M, Giacomini M, Gaillard RC, Grouzmann E 2000 Acceleration of pubertal development following central blockade of the Y1 subtype of neuropeptide Y receptors. Regul Pept 95:47–52 - PubMed
-
- Gonzales C, Voirol MJ, Giacomini M, Gaillard RC, Pedrazzini T, Pralong FP 2004 The neuropeptide Y Y1 receptor mediates NPY-induced inhibition of the gonadotrope axis under poor metabolic conditions. FASEB J 18:137–139 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

