The AIM2 inflammasome is critical for innate immunity to Francisella tularensis
- PMID: 20351693
- PMCID: PMC3111085
- DOI: 10.1038/ni.1859
The AIM2 inflammasome is critical for innate immunity to Francisella tularensis
Abstract
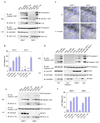
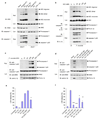
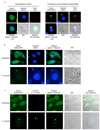
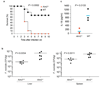
Francisella tularensis, the causative agent of tularemia, infects host macrophages, which triggers production of the proinflammatory cytokines interleukin 1beta (IL-1beta) and IL-18. We elucidate here how host macrophages recognize F. tularensis and elicit this proinflammatory response. Using mice deficient in the DNA-sensing inflammasome component AIM2, we demonstrate here that AIM2 is required for sensing F. tularensis. AIM2-deficient mice were extremely susceptible to F. tularensis infection, with greater mortality and bacterial burden than that of wild-type mice. Caspase-1 activation, IL-1beta secretion and cell death were absent in Aim2(-/-) macrophages in response to F. tularensis infection or the presence of cytoplasmic DNA. Our study identifies AIM2 as a crucial sensor of F. tularensis infection and provides genetic proof of its critical role in host innate immunity to intracellular pathogens.
Figures


Comment in
-
AIMing 2 defend against intracellular pathogens.Nat Immunol. 2010 May;11(5):367-9. doi: 10.1038/ni0510-367. Nat Immunol. 2010. PMID: 20404848 No abstract available.
-
Innate immunity: Ready, AIM, fire!Nat Rev Immunol. 2010 May;10(5):287. doi: 10.1038/nri2771. Nat Rev Immunol. 2010. PMID: 20425914 No abstract available.
Similar articles
-
The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses.Nat Immunol. 2010 May;11(5):395-402. doi: 10.1038/ni.1864. Epub 2010 Mar 28. Nat Immunol. 2010. PMID: 20351692 Free PMC article.
-
Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis.Proc Natl Acad Sci U S A. 2010 May 25;107(21):9771-6. doi: 10.1073/pnas.1003738107. Epub 2010 May 10. Proc Natl Acad Sci U S A. 2010. PMID: 20457908 Free PMC article.
-
Francisella tularensis live vaccine strain folate metabolism and pseudouridine synthase gene mutants modulate macrophage caspase-1 activation.Infect Immun. 2013 Jan;81(1):201-8. doi: 10.1128/IAI.00991-12. Epub 2012 Oct 31. Infect Immun. 2013. PMID: 23115038 Free PMC article.
-
Francisella tularensis: activation of the inflammasome.Ann N Y Acad Sci. 2007 Jun;1105:219-37. doi: 10.1196/annals.1409.005. Epub 2007 Mar 29. Ann N Y Acad Sci. 2007. PMID: 17395724 Review.
-
Francisella Inflammasomes: Integrated Responses to a Cytosolic Stealth Bacterium.Curr Top Microbiol Immunol. 2016;397:229-56. doi: 10.1007/978-3-319-41171-2_12. Curr Top Microbiol Immunol. 2016. PMID: 27460813 Review.
Cited by
-
Salmonella Promotes ASC Oligomerization-dependent Caspase-1 Activation.Immune Netw. 2012 Dec;12(6):284-90. doi: 10.4110/in.2012.12.6.284. Epub 2012 Dec 31. Immune Netw. 2012. PMID: 23396959 Free PMC article.
-
[Caspase-1 regulates autoinflammation in rheumatic diseases].Z Rheumatol. 2016 Apr;75(3):265-75. doi: 10.1007/s00393-016-0077-3. Z Rheumatol. 2016. PMID: 27034076 Review. German.
-
FcγR mediates TLR2- and Syk-dependent NLRP3 inflammasome activation by inactivated Francisella tularensis LVS immune complexes.J Leukoc Biol. 2016 Dec;100(6):1335-1347. doi: 10.1189/jlb.2A1215-555RR. Epub 2016 Jun 30. J Leukoc Biol. 2016. PMID: 27365531 Free PMC article.
-
25-hydroxycholesterol contributes to cerebral inflammation of X-linked adrenoleukodystrophy through activation of the NLRP3 inflammasome.Nat Commun. 2016 Oct 25;7:13129. doi: 10.1038/ncomms13129. Nat Commun. 2016. PMID: 27779191 Free PMC article.
-
Non-transcriptional regulation of NLRP3 inflammasome signaling by IL-4.Immunol Cell Biol. 2015 Jul;93(6):591-9. doi: 10.1038/icb.2014.125. Epub 2015 Jan 20. Immunol Cell Biol. 2015. PMID: 25601272 Free PMC article.
References
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
-
- Kumar H, Kawai T, Akira S. Pathogen recognition in the innate immune response. Biochem J. 2009;420:1–16. - PubMed
-
- Roberts TL, et al. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science. 2009;323:1057–1060. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

