Early Involvement of Immune/Inflammatory Response Genes in Retinal Degeneration in DBA/2J Mice
- PMID: 20352036
- PMCID: PMC2845995
- DOI: 10.4137/oed.s2854
Early Involvement of Immune/Inflammatory Response Genes in Retinal Degeneration in DBA/2J Mice
Abstract
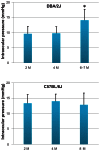
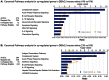
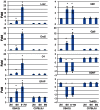
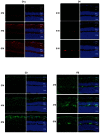
PURPOSE: The DBA/2J (D2) mouse carries mutations in two of its genes, Tyrp1 and Gpnmb. These alterations result in the development of an immune response in the iris, leading to iris atrophy and pigment dispersion. The development of elevated intraocular pressure (IOP) in this model of glaucoma is considered to be a significant factor leading to the death of retinal ganglion cells (RGCs). Changes in gene expression in the retina have already been correlated with the appearance of elevated IOP in the D2 mouse. The purpose of the present study was to determine if any changes in gene expression occur prior to the development of IOP. METHODS: The IOP was measured monthly using a rebound tonometer in D2 and age-matched C57/BL6 (B6) mice (normal controls). D2 animals with normal IOP at 2 and 4 M were used. In addition, mice at the age of 6-7 M were included to look for any trends in gene expression that might develop during the progression of the disease. Separate RNA samples were prepared from each of three individual retinas for each age, and gene expression profiles were determined with the aid of mouse oligonucleotide arrays (Agilent). A subset of genes was examined with the aid of real-time PCR. Immunocytochemistry was used to visualize changes in the retina for some of the gene-products. RESULTS: Four hundred and thirteen oligonucleotide probes were differentially expressed in the retinas of 4 M versus 2 M old D2 mice. The most significantly up-regulated genes (181) were associated with immune responses including interferon signaling, the complement system and the antigen presentation pathway, whereas the down-regulated genes (232) were linked to pathways related to cell death and known neurological diseases/disorders. These particular changes were not revealed in the age-matched B6 mice. By 6 M, when IOP started to increase in many of the D2 mice, more robust changes of these same genes were observed. Changes in the levels of selected genes, representative of different functions/pathways, were validated with RT-PCR, and changes in glial responses were visualized in the retina with immunocytochemistry. CONCLUSIONS: The results showed that the expression of genes related to the immune response and acute stress were altered independently of the development of elevated IOP, and indicated early involvement of the immune system in the onset of the disease. The later development of elevated IOP, observed in this animal model, was coincident with continued changes in expression of genes observed at earlier time points. Further studies are warranted to identify the roles of specific genes identified here with respect to the death of the RGCs.
Figures




References
-
- John SW, Smith RS, Savinova OV, et al. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Invest Ophthalmol Vis Sci. 1998;39:951–62. - PubMed
-
- Anderson MG, Smith RS, Hawes NL, et al. Mutations in genes encoding melanosomal proteins cause pigmentary glaucoma in DBA/2J mice. Nat Genet. 2002;30:81–5. - PubMed
-
- Chang B, Smith RS, Hawes NL, et al. Interacting loci cause severe iris atrophy and glaucoma in DBA/2J mice. Nat Genet. 1999;21:405–9. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

