Versatile virus-like particle carrier for epitope based vaccines
- PMID: 20352110
- PMCID: PMC2843720
- DOI: 10.1371/journal.pone.0009809
Versatile virus-like particle carrier for epitope based vaccines
Abstract
Background: Recombinant proteins and in particular single domains or peptides are often poorly immunogenic unless conjugated to a carrier protein. Virus-like-particles are a very efficient means to confer high immunogenicity to antigens. We report here the development of virus-like-particles (VLPs) derived from the RNA bacteriophage AP205 for epitope-based vaccines.
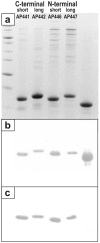
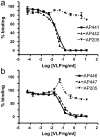
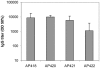
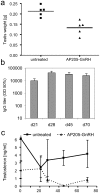
Methodology/principal findings: Peptides of angiotensin II, S.typhi outer membrane protein (D2), CXCR4 receptor, HIV1 Nef, gonadotropin releasing hormone (GnRH), Influenza A M2-protein were fused to either N- or C-terminus of AP205 coat protein. The A205-peptide fusions assembled into VLPs, and peptides displayed on the VLP were highly immunogenic in mice. GnRH fused to the C-terminus of AP205 induced a strong antibody response that inhibited GnRH function in vivo. Exposure of the M2-protein peptide at the N-terminus of AP205 resulted in a strong M2-specific antibody response upon immunization, protecting 100% of mice from a lethal influenza infection.
Conclusions/significance: AP205 VLPs are therefore a very efficient and new vaccine system, suitable for complex and long epitopes, of up to at least 55 amino acid residues in length. AP205 VLPs confer a high immunogenicity to displayed epitopes, as shown by inhibition of endogenous GnRH and protective immunity against influenza infection.
Conflict of interest statement
Figures


Similar articles
-
Bacterial superglue generates a full-length circumsporozoite protein virus-like particle vaccine capable of inducing high and durable antibody responses.Malar J. 2016 Nov 8;15(1):545. doi: 10.1186/s12936-016-1574-1. Malar J. 2016. PMID: 27825348 Free PMC article.
-
Structural basis for the development of avian virus capsids that display influenza virus proteins and induce protective immunity.J Virol. 2015 Mar;89(5):2563-74. doi: 10.1128/JVI.03025-14. Epub 2014 Dec 17. J Virol. 2015. PMID: 25520499 Free PMC article.
-
Mosaic RNA phage VLPs carrying domain III of the West Nile virus E protein.Mol Biotechnol. 2014 May;56(5):459-69. doi: 10.1007/s12033-014-9743-3. Mol Biotechnol. 2014. PMID: 24570176
-
Affinity selection of epitope-based vaccines using a bacteriophage virus-like particle platform.Curr Opin Virol. 2015 Apr;11:76-82. doi: 10.1016/j.coviro.2015.03.005. Epub 2015 Mar 29. Curr Opin Virol. 2015. PMID: 25829254 Free PMC article. Review.
-
Hybrid hepatitis B virus core antigen as a vaccine carrier moiety: I. presentation of foreign epitopes.J Biotechnol. 1996 Jan 26;44(1-3):91-6. doi: 10.1016/0168-1656(95)00118-2. J Biotechnol. 1996. PMID: 8717391 Review.
Cited by
-
Yeast-expressed bacteriophage-like particles for the packaging of nanomaterials.Mol Biotechnol. 2014 Feb;56(2):102-10. doi: 10.1007/s12033-013-9686-0. Mol Biotechnol. 2014. PMID: 23852987
-
Protein-based antigen presentation platforms for nanoparticle vaccines.NPJ Vaccines. 2021 May 13;6(1):70. doi: 10.1038/s41541-021-00330-7. NPJ Vaccines. 2021. PMID: 33986287 Free PMC article. Review.
-
Bacteriophage Virus-Like Particles: Platforms for Vaccine Design.Methods Mol Biol. 2024;2738:411-423. doi: 10.1007/978-1-0716-3549-0_24. Methods Mol Biol. 2024. PMID: 37966612 Review.
-
Virus-like particles as a highly efficient vaccine platform: diversity of targets and production systems and advances in clinical development.Vaccine. 2012 Dec 17;31(1):58-83. doi: 10.1016/j.vaccine.2012.10.083. Epub 2012 Nov 6. Vaccine. 2012. PMID: 23142589 Free PMC article. Review.
-
Preclinical Evaluation of Novel Sterically Optimized VLP-Based Vaccines against All Four DENV Serotypes.Vaccines (Basel). 2024 Aug 1;12(8):874. doi: 10.3390/vaccines12080874. Vaccines (Basel). 2024. PMID: 39204000 Free PMC article.
References
-
- Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. - PubMed
-
- Bachmann MF, Kopf M. The role of B cells in acute and chronic infections. Curr Opin Immunol. 1999;11:332–339. - PubMed
-
- Zinkernagel RM. On natural and artificial vaccinations. Annu Rev Immunol. 2003;21:515–546. - PubMed
-
- Bachmann MF, Dyer MR. Therapeutic vaccination for chronic diseases: a new class of drugs in sight. Nat Rev Drug Discov. 2004;3:81–88. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

