Sensitive detection of gene expression in mycobacteria under replicating and non-replicating conditions using optimized far-red reporters
- PMID: 20352111
- PMCID: PMC2843721
- DOI: 10.1371/journal.pone.0009823
Sensitive detection of gene expression in mycobacteria under replicating and non-replicating conditions using optimized far-red reporters
Abstract
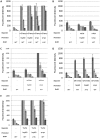
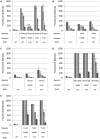
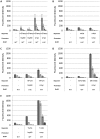
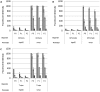
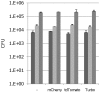
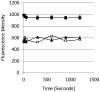
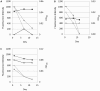
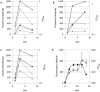
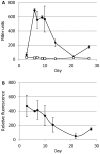
Fluorescent reporter proteins have proven useful for imaging techniques in many organisms. We constructed optimized expression systems for several fluorescent proteins from the far-red region of the spectrum and analyzed their utility in several mycobacterial species. Plasmids expressing variants of the Discosoma Red fluorescent protein (DsRed) from the Mycobacterium bovis hsp60 promoter were unstable; in contrast expression from the Mycobacterium smegmatis rpsA promoter was stable. In Mycobacterium tuberculosis expression of several of the far-red reporters was readily visualised by eye and three reporters (mCherry, tdTomato, and Turbo-635) fluoresced at a high intensity. Strains expressing mCherry showed no fitness defects in vitro or in macrophages. Treatment of cells with antibiotics demonstrated that mCherry could also be used as a reporter for cell death, since fluorescence decreased in the presence of a bactericidal compound, but remained stable in the presence of a bacteriostatic compound. mCherry was functional under hypoxic conditions; using mCherry we demonstrated that the P(mtbB) is expressed early in hypoxia and progressively down-regulated. mCherry and other far-red fluorescent proteins will have multiple uses in investigating the biology of mycobacteria, particularly under non-replicating, or low cell density conditions, as well as providing a novel means of detecting cell death rapidly.
Conflict of interest statement
Figures


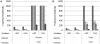

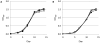
References
-
- Coons AH, Creech HJ, Jones RN, Berliner E. The demonstration of pneumococcal antigen in tissues by the use of fluorescent antibody. J Immunol. 1942;45:159–170.
-
- Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. - PubMed
-
- Carroll P, James J. Assaying promoter activity using LacZ and GFP as reporters. In: Parish T, Brown AC, editors. Mycobacteria Protocols: Humana Press; 2009. pp. 265–278. - PubMed
-
- Dhandayuthapani S, Via LE, Thomas CA, Horowitz PM, Deretic D, et al. Green fluorescent protein as a marker for gene expression and cell biology of mycobacterial interactions with macrophages. Mol Microbiol. 1995;17:901–912. - PubMed
-
- McKinney JD, Honer zu Bentrup K, Munoz-Elias EJ, Miczak A, Chen B, et al. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature. 2000;406:735–738. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

