Depo-provera treatment does not abrogate protection from intravenous SIV challenge in female macaques immunized with an attenuated AIDS virus
- PMID: 20352116
- PMCID: PMC2843738
- DOI: 10.1371/journal.pone.0009814
Depo-provera treatment does not abrogate protection from intravenous SIV challenge in female macaques immunized with an attenuated AIDS virus
Abstract
Background: In a previous study, progesterone treatment of female monkeys immunized with live, attenuated SHIV89.6 abrogated the generally consistent protection from vaginal simian immunodeficiency virus (SIV) challenge. The mechanisms responsible for the loss of protection remain to be defined. The objective of the present study was to determine whether Depo-Provera administration alters protection from intravenous SIV challenge in SHIV-immunized female macaques.
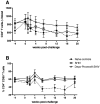
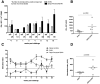
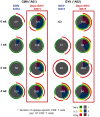
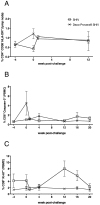
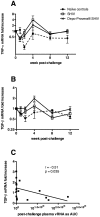
Methods and findings: Two groups of female macaques were immunized with attenuated SHIV89.6 and then challenged intravenously with SIVmac239. Four weeks before challenge, one animal group was treated with Depo-Provera, a commonly used injectable contraceptive progestin. As expected, SHIV-immunized monkeys had significantly lower peak and set-point plasma viral RNA levels compared to naïve controls, but in contrast to previously published findings with vaginal SIV challenge, the Depo-Provera SHIV-immunized animals controlled SIV replication to a similar, or even slightly greater, degree than did the untreated SHIV-immunized animals. Control of viral replication from week 4 to week 20 after challenge was more consistent in the progesterone-treated, SHIV-immunized animals than in untreated, SHIV-immunized animals. Although levels of interferon-gamma production were similar, the SIV-specific CD8(+) T cells of progesterone-treated animals expressed more functions than the anti-viral CD8(+) T cells from untreated animals.
Conclusions: Depo-Provera did not diminish the control of viral replication after intravenous SIV challenge in female macaques immunized with a live-attenuated lentivirus. This result contrasts with the previously reported effect of Depo-Provera(R) on protection from vaginal SIV challenge and strongly implies that the decreased protection from vaginal challenge is due to effects of progesterone on the genital tract rather than to systemic effects. Further, these results demonstrate that the effects of hormonal contraceptives on vaccine efficacy need to be considered in the context of testing and use of an AIDS vaccine.
Conflict of interest statement
Figures

Similar articles
-
Depo-Provera abrogates attenuated lentivirus-induced protection in male rhesus macaques challenged intravenously with pathogenic SIVmac239.J Med Primatol. 2007 Aug;36(4-5):266-75. doi: 10.1111/j.1600-0684.2007.00244.x. J Med Primatol. 2007. PMID: 17669215 Free PMC article.
-
Abrogation of attenuated lentivirus-induced protection in rhesus macaques by administration of depo-provera before intravaginal challenge with simian immunodeficiency virus mac239.J Infect Dis. 2004 Nov 1;190(9):1697-705. doi: 10.1086/424600. Epub 2004 Sep 24. J Infect Dis. 2004. PMID: 15478078 Free PMC article.
-
With minimal systemic T-cell expansion, CD8+ T Cells mediate protection of rhesus macaques immunized with attenuated simian-human immunodeficiency virus SHIV89.6 from vaginal challenge with simian immunodeficiency virus.J Virol. 2008 Nov;82(22):11181-96. doi: 10.1128/JVI.01433-08. Epub 2008 Sep 10. J Virol. 2008. PMID: 18787003 Free PMC article.
-
Antiviral CD8+ T cells in the genital tract control viral replication and delay progression to AIDS after vaginal SIV challenge in rhesus macaques immunized with virulence attenuated SHIV 89.6.J Intern Med. 2009 Jan;265(1):67-77. doi: 10.1111/j.1365-2796.2008.02051.x. J Intern Med. 2009. PMID: 19093961 Free PMC article. Review.
-
Immune mechanisms associated with protection from vaginal SIV challenge in rhesus monkeys infected with virulence-attenuated SHIV 89.6.J Med Primatol. 2005 Oct;34(5-6):271-81. doi: 10.1111/j.1600-0684.2005.00125.x. J Med Primatol. 2005. PMID: 16128922 Free PMC article. Review.
Cited by
-
Animal models for microbicide studies.Curr HIV Res. 2012 Jan 1;10(1):79-87. doi: 10.2174/157016212799304715. Curr HIV Res. 2012. PMID: 22264049 Free PMC article. Review.
-
Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody.Proc Natl Acad Sci U S A. 2011 Jul 5;108(27):11181-6. doi: 10.1073/pnas.1103012108. Epub 2011 Jun 20. Proc Natl Acad Sci U S A. 2011. PMID: 21690411 Free PMC article.
-
Invaplex functions as an intranasal adjuvant for subunit and DNA vaccines co-delivered in the nasal cavity of nonhuman primates.Vaccine X. 2021 Jun 24;8:100105. doi: 10.1016/j.jvacx.2021.100105. eCollection 2021 Aug. Vaccine X. 2021. PMID: 34258576 Free PMC article.
-
Depot medroxyprogesterone acetate increases immune cell numbers and activation markers in human vaginal mucosal tissues.AIDS Res Hum Retroviruses. 2013 Mar;29(3):592-601. doi: 10.1089/aid.2012.0271. Epub 2012 Nov 28. AIDS Res Hum Retroviruses. 2013. PMID: 23189932 Free PMC article.
-
Dendritic cell activation and memory cell development are impaired among mice administered medroxyprogesterone acetate prior to mucosal herpes simplex virus type 1 infection.J Immunol. 2012 Oct 1;189(7):3449-61. doi: 10.4049/jimmunol.1103054. Epub 2012 Aug 31. J Immunol. 2012. PMID: 22942424 Free PMC article.
References
-
- Abel K, Compton L, Rourke T, Montefiori D, Lu D, et al. Simian-human immunodeficiency virus SHIV89.6-induced protection against intravaginal challenge with pathogenic SIVmac239 is independent of the route of immunization and is associated with a combination of cytotoxic T-lymphocyte and alpha interferon responses. J Virol. 2003;77:3099–3118. - PMC - PubMed
-
- Moodley D, Esterhuizen TM, Pather T, Chetty V, Ngaleka L. High HIV incidence during pregnancy: compelling reason for repeat HIV testing. AIDS. 2009;23:1255–1259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

