Stress-induced sphingolipid signaling: role of type-2 neutral sphingomyelinase in murine cell apoptosis and proliferation
- PMID: 20352118
- PMCID: PMC2843740
- DOI: 10.1371/journal.pone.0009826
Stress-induced sphingolipid signaling: role of type-2 neutral sphingomyelinase in murine cell apoptosis and proliferation
Expression of concern in
-
Expression of Concern: Stress-Induced Sphingolipid Signaling: Role of Type-2 Neutral Sphingomyelinase in Murine Cell Apoptosis and Proliferation.PLoS One. 2018 Dec 10;13(12):e0208866. doi: 10.1371/journal.pone.0208866. eCollection 2018. PLoS One. 2018. PMID: 30532272 Free PMC article. No abstract available.
Abstract
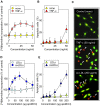
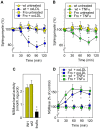
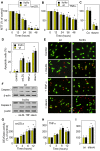
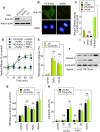
Background: Sphingomyelin hydrolysis in response to stress-inducing agents, and subsequent ceramide generation, are implicated in various cellular responses, including apoptosis, inflammation and proliferation, depending on the nature of the different acidic or neutral sphingomyelinases. This study was carried out to investigate whether the neutral Mg(2+)-dependent neutral sphingomyelinase-2 (nSMase2) plays a role in the cellular signaling evoked by TNFalpha and oxidized LDLs, two stress-inducing agents, which are mitogenic at low concentrations and proapoptotic at higher concentrations.
Methodology and principal findings: For this purpose, we used nSMase2-deficient cells from homozygous fro/fro (fragilitas ossium) mice and nSMase2-deficient cells reconstituted with a V5-tagged nSMase2. We report that the genetic defect of nSMase2 (in fibroblasts from fro/fro mice) does not alter the TNFalpha and oxidized LDLs-mediated apoptotic response. Likewise, the hepatic toxicity of TNFalpha is similar in wild type and fro mice, thus is independent of nSMase2 activation. In contrast, the mitogenic response elicited by low concentrations of TNFalpha and oxidized LDLs (but not fetal calf serum) requires nSMase2 activation.
Conclusion and significance: nSMase2 activation is not involved in apoptosis mediated by TNFalpha and oxidized LDLs in murine fibroblasts, and in the hepatotoxicity of TNFalpha in mice, but is required for the mitogenic response to stress-inducing agents.
Conflict of interest statement
Figures
Similar articles
-
Hyaluronan synthase-2 upregulation protects smpd3-deficient fibroblasts against cell death induced by nutrient deprivation, but not against apoptosis evoked by oxidized LDL.Redox Biol. 2015;4:118-26. doi: 10.1016/j.redox.2014.12.004. Epub 2014 Dec 16. Redox Biol. 2015. PMID: 25555205 Free PMC article.
-
Biochemical properties of mammalian neutral sphingomyelinase 2 and its role in sphingolipid metabolism.J Biol Chem. 2003 Apr 18;278(16):13775-83. doi: 10.1074/jbc.M212262200. Epub 2003 Feb 3. J Biol Chem. 2003. PMID: 12566438
-
A signaling cascade mediated by ceramide, src and PDGFRβ coordinates the activation of the redox-sensitive neutral sphingomyelinase-2 and sphingosine kinase-1.Biochim Biophys Acta. 2013 Aug;1831(8):1344-56. doi: 10.1016/j.bbalip.2013.04.014. Epub 2013 May 4. Biochim Biophys Acta. 2013. PMID: 23651497
-
Sphingolipid metabolism and neutral sphingomyelinases.Handb Exp Pharmacol. 2013;(215):57-76. doi: 10.1007/978-3-7091-1368-4_3. Handb Exp Pharmacol. 2013. PMID: 23579449 Free PMC article. Review.
-
Neutral sphingomyelinase-2 and cardiometabolic diseases.Obes Rev. 2021 Aug;22(8):e13248. doi: 10.1111/obr.13248. Epub 2021 Mar 18. Obes Rev. 2021. PMID: 33738905 Free PMC article. Review.
Cited by
-
The Effect of Vitamin D3 and Silver Nanoparticles on HaCaT Cell Viability.Int J Mol Sci. 2022 Jan 26;23(3):1410. doi: 10.3390/ijms23031410. Int J Mol Sci. 2022. PMID: 35163332 Free PMC article.
-
Expression of Concern: Stress-Induced Sphingolipid Signaling: Role of Type-2 Neutral Sphingomyelinase in Murine Cell Apoptosis and Proliferation.PLoS One. 2018 Dec 10;13(12):e0208866. doi: 10.1371/journal.pone.0208866. eCollection 2018. PLoS One. 2018. PMID: 30532272 Free PMC article. No abstract available.
-
Sphingolipids in Atherosclerosis: Chimeras in Structure and Function.Int J Mol Sci. 2022 Oct 8;23(19):11948. doi: 10.3390/ijms231911948. Int J Mol Sci. 2022. PMID: 36233252 Free PMC article. Review.
-
Off-target function of the Sonic hedgehog inhibitor cyclopamine in mediating apoptosis via nitric oxide-dependent neutral sphingomyelinase 2/ceramide induction.Mol Cancer Ther. 2012 May;11(5):1092-102. doi: 10.1158/1535-7163.MCT-11-0705. Epub 2012 Mar 27. Mol Cancer Ther. 2012. PMID: 22452947 Free PMC article.
-
Sphingolipids in neurodegeneration (with focus on ceramide and S1P).Adv Biol Regul. 2018 Dec;70:51-64. doi: 10.1016/j.jbior.2018.09.013. Epub 2018 Sep 22. Adv Biol Regul. 2018. PMID: 30287225 Free PMC article. Review.
References
-
- Hannun YA. Functions of ceramide in coordinating cellular responses to stress. Science. 1996;274:1855–1859. - PubMed
-
- Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. - PubMed
-
- Kolesnick RN, Kronke M. Regulation of ceramide production and apoptosis. Annu Rev Physiol. 1998;60:643–665. - PubMed
-
- Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. - PubMed
-
- Clarke CJ, Hannun YA. Neutral sphingomyelinases and nSMase2: bridging the gaps. Biochim Biophys Acta. 2006;1758:1893–1901. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

