Using In Vivo Electrochemistry to Study the Physiological Effects of Cocaine and Other Stimulants on the Drosophila melanogaster Dopamine Transporter
- PMID: 20352129
- PMCID: PMC2843917
- DOI: 10.1021/cn900017w
Using In Vivo Electrochemistry to Study the Physiological Effects of Cocaine and Other Stimulants on the Drosophila melanogaster Dopamine Transporter
Abstract
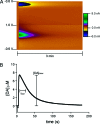
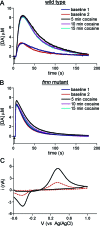
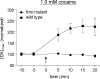
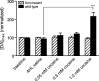
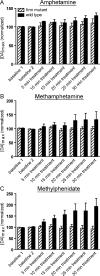
Dopamine neurotransmission is thought to play a critical role in addiction reinforcing mechanisms of drugs of abuse. Electrochemical techniques have been employed extensively for monitoring in vivo dopamine changes in the brains of model organisms including rats, mice, and primates. Here, we investigated the effects of several stimulants on dopamine clearance using recently developed microanalytical tools for in vivo electrochemical measurements of dopamine in the central nervous system of Drosophila melanogaster. A cylindrical carbon-fiber microelectrode was placed in the protocerebral anterior medial region of the Drosophila brain (an area dense with dopamine neurons) while a micropipette injector was positioned to exogenously apply dopamine. Background-subtracted fast-scan cyclic voltammetry was carried out to quantify changes in dopamine concentration in the adult fly brain. Clearance of exogenously applied dopamine was significantly decreased in the protocerebral anterior medial area of the wild-type fly following treatment with cocaine, amphetamine, methamphetamine, or methylphenidate. In contrast, dopamine uptake remained unchanged when identical treatments were employed in fumin mutant flies that lack functional dopamine transporters. Our in vivo results support in vitro binding affinity studies that predict these four stimulants effectively block normal Drosophila dopamine transporter function. Furthermore, we found 10 muM to be a sufficient physiological cocaine concentration to significantly alter dopamine transporter uptake in the Drosophila central nervous system. Taken together, these data indicate dopamine uptake in the Drosophila brain is decreased by psychostimulants as observed in mammals. This validates the use of Drosophila as a model system for future studies into the cellular and molecular mechanisms underlying drug addiction in humans.
Figures
Similar articles
-
In vivo electrochemical measurements of exogenously applied dopamine in Drosophila melanogaster.Anal Chem. 2009 Mar 1;81(5):1848-54. doi: 10.1021/ac802297b. Anal Chem. 2009. PMID: 19192966 Free PMC article.
-
Kinetics of the dopamine transporter in Drosophila larva.ACS Chem Neurosci. 2013 May 15;4(5):832-7. doi: 10.1021/cn400019q. Epub 2013 Apr 26. ACS Chem Neurosci. 2013. PMID: 23600464 Free PMC article.
-
Oral administration of methylphenidate blocks the effect of cocaine on uptake at the Drosophila dopamine transporter.ACS Chem Neurosci. 2013 Apr 17;4(4):566-74. doi: 10.1021/cn3002009. Epub 2013 Feb 25. ACS Chem Neurosci. 2013. PMID: 23402315 Free PMC article.
-
Drosophila as a Model System for Neurotransmitter Measurements.ACS Chem Neurosci. 2018 Aug 15;9(8):1872-1883. doi: 10.1021/acschemneuro.7b00456. Epub 2018 Feb 20. ACS Chem Neurosci. 2018. PMID: 29411967 Free PMC article. Review.
-
Psychostimulants affect dopamine transmission through both dopamine transporter-dependent and independent mechanisms.Eur J Pharmacol. 2015 Oct 5;764:562-570. doi: 10.1016/j.ejphar.2015.07.044. Epub 2015 Jul 21. Eur J Pharmacol. 2015. PMID: 26209364 Free PMC article. Review.
Cited by
-
Domain-dependent effects of DAT inhibition in the rat dorsal striatum.J Neurochem. 2012 Jul;122(2):283-94. doi: 10.1111/j.1471-4159.2012.07774.x. Epub 2012 May 28. J Neurochem. 2012. PMID: 22548305 Free PMC article.
-
Distinct and Dynamic Changes in the Temporal Profiles of Neurotransmitters in Drosophila melanogaster Brain following Volatilized Cocaine or Methamphetamine Administrations.Pharmaceuticals (Basel). 2023 Oct 19;16(10):1489. doi: 10.3390/ph16101489. Pharmaceuticals (Basel). 2023. PMID: 37895961 Free PMC article.
-
Freeze-drying as sample preparation for micellar electrokinetic capillary chromatography-electrochemical separations of neurochemicals in Drosophila brains.Anal Chem. 2013 Mar 5;85(5):2841-6. doi: 10.1021/ac303377x. Epub 2013 Feb 22. Anal Chem. 2013. PMID: 23387977 Free PMC article.
-
Big Lessons from Tiny Flies: Drosophila melanogaster as a Model to Explore Dysfunction of Dopaminergic and Serotonergic Neurotransmitter Systems.Int J Mol Sci. 2018 Jun 16;19(6):1788. doi: 10.3390/ijms19061788. Int J Mol Sci. 2018. PMID: 29914172 Free PMC article. Review.
-
Electrochemical Analysis of Neurotransmitters.Annu Rev Anal Chem (Palo Alto Calif). 2015;8:239-61. doi: 10.1146/annurev-anchem-071114-040426. Epub 2015 May 4. Annu Rev Anal Chem (Palo Alto Calif). 2015. PMID: 25939038 Free PMC article. Review.
References
-
- Chen N.; Reith M. E. A. (2000) Structure and function of the dopamine transporter. Eur. J. Pharmacol. 405, 329–339. - PubMed
-
- Mortensen O. V.; Amara S. G. (2003) Dynamic regulation of the dopamine transporter. Eur. J. Pharmacol. 479, 159–170. - PubMed
-
- Kuhar M. J.; Ritz M. C.; Boja J. W. (1991) The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci. 14, 299–302. - PubMed
-
- Giros B.; Jaber M.; Jones S. R.; Wightman R. M.; Caron M. G. (1996) Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 379, 606–612. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

