Metabolism and function of phenazines in bacteria: impacts on the behavior of bacteria in the environment and biotechnological processes
- PMID: 20352425
- PMCID: PMC2858273
- DOI: 10.1007/s00253-010-2509-3
Metabolism and function of phenazines in bacteria: impacts on the behavior of bacteria in the environment and biotechnological processes
Abstract
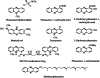
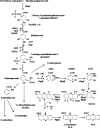
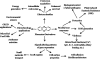
Phenazines constitute a large group of nitrogen-containing heterocyclic compounds produced by a diverse range of bacteria. Both natural and synthetic phenazine derivatives are studied due their impacts on bacterial interactions and biotechnological processes. Phenazines serve as electron shuttles to alternate terminal acceptors, modify cellular redox states, act as cell signals that regulate patterns of gene expression, contribute to biofilm formation and architecture, and enhance bacterial survival. Phenazines have diverse effects on eukaryotic hosts and host tissues, including the modification of multiple host cellular responses. In plants, phenazines also may influence growth and elicit induced systemic resistance. Here, we discuss emerging evidence that phenazines play multiple roles for the producing organism and contribute to their behavior and ecological fitness.
Figures
Similar articles
-
Interdependency of Respiratory Metabolism and Phenazine-Associated Physiology in Pseudomonas aeruginosa PA14.J Bacteriol. 2020 Jan 29;202(4):e00700-19. doi: 10.1128/JB.00700-19. Print 2020 Jan 29. J Bacteriol. 2020. PMID: 31767778 Free PMC article.
-
Nitrate Reduction Stimulates and Is Stimulated by Phenazine-1-Carboxylic Acid Oxidation by Citrobacter portucalensis MBL.mBio. 2021 Aug 31;12(4):e0226521. doi: 10.1128/mBio.02265-21. Epub 2021 Aug 31. mBio. 2021. PMID: 34465028 Free PMC article.
-
Pseudomonas aeruginosa PumA acts on an endogenous phenazine to promote self-resistance.Microbiology (Reading). 2018 May;164(5):790-800. doi: 10.1099/mic.0.000657. Epub 2018 Apr 9. Microbiology (Reading). 2018. PMID: 29629858 Free PMC article.
-
Extracellular electron transfer.Cell Mol Life Sci. 2001 Oct;58(11):1562-71. doi: 10.1007/PL00000796. Cell Mol Life Sci. 2001. PMID: 11706984 Free PMC article. Review.
-
Recent insights into the diversity, frequency and ecological roles of phenazines in fluorescent Pseudomonas spp.Environ Microbiol. 2013 Mar;15(3):675-86. doi: 10.1111/j.1462-2920.2012.02846.x. Epub 2012 Aug 13. Environ Microbiol. 2013. PMID: 22882648 Review.
Cited by
-
Pseudomonas putida-a versatile host for the production of natural products.Appl Microbiol Biotechnol. 2015 Aug;99(15):6197-214. doi: 10.1007/s00253-015-6745-4. Epub 2015 Jun 23. Appl Microbiol Biotechnol. 2015. PMID: 26099332 Free PMC article. Review.
-
Metabolic reconstruction of Pseudomonas chlororaphis ATCC 9446 to understand its metabolic potential as a phenazine-1-carboxamide-producing strain.Appl Microbiol Biotechnol. 2020 Dec;104(23):10119-10132. doi: 10.1007/s00253-020-10913-4. Epub 2020 Sep 28. Appl Microbiol Biotechnol. 2020. PMID: 32984920
-
Fluorescent redox-dependent labeling of lipid droplets in cultured cells by reduced phenazine methosulfate.Heliyon. 2020 Jun 13;6(6):e04182. doi: 10.1016/j.heliyon.2020.e04182. eCollection 2020 Jun. Heliyon. 2020. PMID: 32566788 Free PMC article.
-
Non-canonical pattern recognition of a pathogen-derived metabolite by a nuclear hormone receptor identifies virulent bacteria in C. elegans.Immunity. 2023 Apr 11;56(4):768-782.e9. doi: 10.1016/j.immuni.2023.01.027. Epub 2023 Feb 17. Immunity. 2023. PMID: 36804958 Free PMC article.
-
Complete Genome Sequence of the Plant Growth-Promoting Bacterium Hartmannibacter diazotrophicus Strain E19T.Int J Genomics. 2019 Sep 9;2019:7586430. doi: 10.1155/2019/7586430. eCollection 2019. Int J Genomics. 2019. PMID: 31583244 Free PMC article.
References
-
- Beifuss U, Tietze M. Methanophenzine and other natural biologically active phenazines. Top Curr Chem. 2005;244:77–113.
-
- Chen JP, Xiao-Chang CL (2004) Organic light-emitting device having phenanthroline-fused phenazine. US Patent 6713781
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

