Imaging and photodynamic therapy: mechanisms, monitoring, and optimization
- PMID: 20353192
- PMCID: PMC2896821
- DOI: 10.1021/cr900300p
Imaging and photodynamic therapy: mechanisms, monitoring, and optimization
Figures







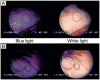

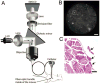

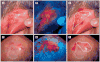
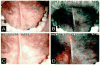


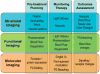



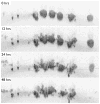



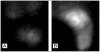
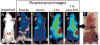




References
-
- Fitzpatrick TB, Pathak MA. J Invest Dermatol. 1959;32:229. - PubMed
-
- Epstein JH. N Engl J Med. 1990;322:1149. - PubMed
-
- Lipson RL, Baldes EJ, Olsen AM. J Natl Cancer Inst. 1961;26:1. - PubMed
-
- Kessel D. Photochem Photobiol. 1986;44:193. - PubMed
-
- Dougherty TJ. J Clin Laser Med Surg. 1996;14:219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

