Moving research to patient applications through commercialization: understanding and evaluating the role of intellectual property
- PMID: 20353687
- PMCID: PMC2846000
Moving research to patient applications through commercialization: understanding and evaluating the role of intellectual property
Abstract
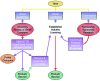
The advancement of research from discovery to the delivery of medical care can be limited without the support of industry to sponsor its continued development. Federal government financial support is generally crucial in early-stage development through funding from the NIH, National Science Foundation, and other federal agencies; however, government support generally stops shortly after basic research discoveries have been reported. Much of the cessation of financial support derives from the government's regulatory responsibilities, as sponsoring the commercialization of a product conflicts with regulation of the approval for clinical use of a drug or device. Furthermore, differences in goals, resources, and flexibility render government, as compared with private industry, inefficient and less responsive to market demands with regard to stream-lining the development of and enhancing the quality of products and services offered. Thus, industry and private investment provide the bridge that converts new discoveries into healthcare products that are available to consumers and patients. This conversion occurs through commercialization, which involves both high risks and high rewards. Taking advantage of the commercialization option for research development requires an understanding of the technology transfer process. This article reviews 5 topics: 1) industry motivation to invest in academic research; 2) institutional considerations in partnering with industry; 3) academia's interactions with inventors in the commercialization process; 4) the research institution's route to commercialization, and 5) the role of intellectual property and commercialization in the advancement of healthcare.
Figures



References
-
- AUTM Better World Project 2008. The Better World report: part one. The art of collaboration: the relationships that bring academic innovations to the marketplace. Deerfield (IL): Association of University Technology Managers
-
- Berneman LP, Denis KA. 2002. University licensing trends and intellectual capital, p 234–235 : Goldscheider The LESI guide to licensing best practices, strategic issues and contemporary realities. New York (NY): John Wiley and Sons
-
- Bloomberg CA, Ayazi AF. 2007. Patent forfeiture under the Bayh–Dole Act. Health Care Compliance Assoc 9:40–41, 43, 46–47, 49, 51
-
- California Institute of Technology, Cornell University, Harvard University, Massachusetts Institute of Technology, Stanford University, University of California, University of Illinois, Chicago, University of Illinois, Urbana-Champaign, University of Washington, Wisconsin Alumni Research Foundation, Yale University, and the Association of American Medical Colleges (AAMC) 2007. In the public interest: nine points to consider in licensing university technology.
-
- Centers for Medicare & Medicaid Services [Internet]. National health expenditure projections 2007 – 2017. [Cited 13 Feb 2009]. Available from http://www.cms.hhs.gov/NationalHealthExpendData/Downloads/proj2007.pdf - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
