Cis and trans actions of the cholinesterase-like domain within the thyroglobulin dimer
- PMID: 20353937
- PMCID: PMC2878521
- DOI: 10.1074/jbc.M110.111641
Cis and trans actions of the cholinesterase-like domain within the thyroglobulin dimer
Abstract
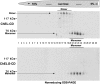
Thyroglobulin (Tg, precursor for thyroid hormone synthesis) is a large secreted glycoprotein composed of upstream regions I-II-III, followed by the approximately 570 residue cholinesterase-like (ChEL) domain. ChEL has two identified functions: 1) homodimerization, and 2) binding to I-II-III that facilitates I-II-III oxidative maturation required for intracellular protein transport. Like its homologs in the acetylcholinesterase (AChE) family, ChEL possesses two carboxyl-terminal alpha-helices. We find that a Tg-AChE chimera (swapping AChE in place of ChEL) allows for dimerization with monomeric AChE, proving exposure of the carboxyl-terminal helices within the larger context of Tg. Further, we establish that perturbing trans-helical interaction blocks homodimerization of the Tg ChEL domain. Additionally, ChEL can associate with neuroligins (a related family of cholinesterase-like proteins), demonstrating potential for Tg cross-dimerization between non-identical partners. Indeed, when mutant rdw-Tg (Tg-G2298R, defective for protein secretion) is co-expressed with wild-type Tg, the two proteins cross-dimerize and secretion of rdw-Tg is partially restored. Moreover, we find that AChE and soluble neuroligins also can bind to the upstream Tg regions I-II-III; however, they cannot rescue secretion, because they cannot facilitate oxidative maturation of I-II-III. These data suggest that specific properties of distinct Tg ChEL mutants may result in distinct patterns of Tg monomer folding, cross-dimerization with wild-type Tg, and variable secretion behavior in heterozygous patients.
Figures

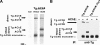



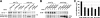


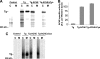
Similar articles
-
The cholinesterase-like domain, essential in thyroglobulin trafficking for thyroid hormone synthesis, is required for protein dimerization.J Biol Chem. 2009 May 8;284(19):12752-61. doi: 10.1074/jbc.M806898200. Epub 2009 Mar 9. J Biol Chem. 2009. PMID: 19276074 Free PMC article.
-
The cholinesterase-like domain of thyroglobulin functions as an intramolecular chaperone.J Clin Invest. 2008 Aug;118(8):2950-8. doi: 10.1172/JCI35164. J Clin Invest. 2008. PMID: 18596923 Free PMC article.
-
The acetylcholinesterase homology region is essential for normal conformational maturation and secretion of thyroglobulin.J Biol Chem. 2004 Apr 23;279(17):17085-9. doi: 10.1074/jbc.M314042200. Epub 2004 Feb 5. J Biol Chem. 2004. PMID: 14764582
-
Thyroglobulin From Molecular and Cellular Biology to Clinical Endocrinology.Endocr Rev. 2016 Feb;37(1):2-36. doi: 10.1210/er.2015-1090. Epub 2015 Nov 23. Endocr Rev. 2016. PMID: 26595189 Free PMC article. Review.
-
The origin of the molecular diversity and functional anchoring of cholinesterases.Neurosignals. 2002 May-Jun;11(3):130-43. doi: 10.1159/000065054. Neurosignals. 2002. PMID: 12138250 Review.
Cited by
-
From Split to Sibenik: the tortuous pathway in the cholinesterase field.Chem Biol Interact. 2010 Sep 6;187(1-3):3-9. doi: 10.1016/j.cbi.2010.05.005. Epub 2010 May 20. Chem Biol Interact. 2010. PMID: 20493179 Free PMC article.
-
Lessons from animal models of endocrine disorders caused by defects of protein folding in the secretory pathway.Mol Cell Endocrinol. 2020 Jan 1;499:110613. doi: 10.1016/j.mce.2019.110613. Epub 2019 Oct 9. Mol Cell Endocrinol. 2020. PMID: 31605742 Free PMC article. Review.
-
Relationship between the dimerization of thyroglobulin and its ability to form triiodothyronine.J Biol Chem. 2018 Mar 30;293(13):4860-4869. doi: 10.1074/jbc.RA118.001786. Epub 2018 Feb 12. J Biol Chem. 2018. PMID: 29440273 Free PMC article.
-
Transient covalent interactions of newly synthesized thyroglobulin with oxidoreductases of the endoplasmic reticulum.J Biol Chem. 2014 Apr 18;289(16):11488-11496. doi: 10.1074/jbc.M113.520767. Epub 2014 Mar 5. J Biol Chem. 2014. PMID: 24599957 Free PMC article.
-
Anaplastic thyroid carcinoma: vimentin segregates at the invasive front of tumors in a murine xenograft model.Histochem Cell Biol. 2024 Nov 18;163(1):6. doi: 10.1007/s00418-024-02329-2. Histochem Cell Biol. 2024. PMID: 39557701
References
-
- Di Jeso B., Arvan P. (2004) in The Thyroid (Braverman L. E., Utiger R. eds) 9th Ed., pp. 77–95, Lippincott Williams & Wilkins, Philadelphia
-
- Taurog A. (1999) Biochimie 81, 557–562 - PubMed
-
- Marriq C., Lejeune P. J., Venot N., Vinet L. (1991) Mol. Cell. Endocrinol. 81, 155–164 - PubMed
-
- Dunn A. D., Corsi C. M., Myers H. E., Dunn J. T. (1998) J. Biol. Chem. 273, 25223–25229 - PubMed
-
- Lamas L., Taurog A. (1977) Endocrinology 100, 1129–1136 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

