The catecholaminergic polymorphic ventricular tachycardia mutation R33Q disrupts the N-terminal structural motif that regulates reversible calsequestrin polymerization
- PMID: 20353949
- PMCID: PMC2878038
- DOI: 10.1074/jbc.M109.096354
The catecholaminergic polymorphic ventricular tachycardia mutation R33Q disrupts the N-terminal structural motif that regulates reversible calsequestrin polymerization
Abstract
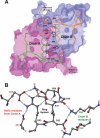
Calsequestrin undergoes dynamic polymerization with increasing calcium concentration by front-to-front dimerization and back-to-back packing, forming wire-shaped structures. A recent finding that point mutation R33Q leads to lethal catecholaminergic polymorphic ventricular tachycardia (CPVT) implies a crucial role for the N terminus. In this study, we demonstrate that this mutation resides in a highly conserved alternately charged residue cluster (DGKDR; cluster 1) in the N-terminal end of calsequestrin. We further show that this cluster configures itself as a ring system and that the dipolar arrangement within the cluster brings about a critical conformational flip of Lys(31)-Asp(32) essential for dimer stabilization by formation of a H-bond network. We additionally show that Ca(2+)-induced calsequestrin aggregation is nonlinear and reversible and can regain the native conformation by Ca(2+) chelation with EGTA. This study suggests that cluster 1 works as a molecular switch and governs the bidirectional transition between the CASQ2 monomer and dimer. We further demonstrate that mutations disrupting the alternating charge pattern of the cluster, including R33Q, impair Ca(2+)-CASQ2 interaction, leading to altered polymerization-depolymerization dynamics. This study provides new mechanistic insight into the functional effects of the R33Q mutation and its potential role in CPVT.
Figures





References
-
- Bers D. M. (1997) Basic Res. Cardiol. 92, Suppl. 1, 1–10 - PubMed
-
- MacLennan D. H., Campbell K. P., Reithmeier R. A. F. (1983) in Calcium and Cell Function (Cheung W. Y. ed) Vol. IV, pp. 151–173, Academic Press, Inc., New York
-
- Slupsky J. R., Ohnishi M., Carpenter M. R., Reithmeier R. A. (1987) Biochemistry 26, 6539–6544 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

