AP-1 (Fra-1/c-Jun)-mediated induction of expression of matrix metalloproteinase-2 is required for 15S-hydroxyeicosatetraenoic acid-induced angiogenesis
- PMID: 20353950
- PMCID: PMC2878006
- DOI: 10.1074/jbc.M110.106187
AP-1 (Fra-1/c-Jun)-mediated induction of expression of matrix metalloproteinase-2 is required for 15S-hydroxyeicosatetraenoic acid-induced angiogenesis
Abstract
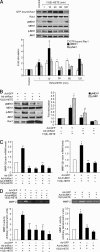
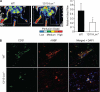
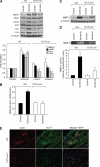
To understand the involvement of matrix metalloproteinases (MMPs) in 15(S)-hydroxyeicosatetraenoic acid (15(S)-HETE)-induced angiogenesis, we have studied the role of MMP-2. 15(S)-HETE induced MMP-2 expression and activity in a time-dependent manner in human dermal microvascular endothelial cells (HDMVECs). Inhibition of MMP-2 activity or depletion of its levels attenuated 15(S)-HETE-induced HDMVEC migration, tube formation, and Matrigel plug angiogenesis. 15(S)-HETE also induced Fra-1 and c-Jun expression in a Rac1-MEK1-JNK1-dependent manner. In addition, 15(S)-HETE-induced MMP-2 expression and activity were mediated by Rac1-MEK1-JNK1-dependent activation of AP-1 (Fra-1/c-Jun). Cloning and site-directed mutagenesis of MMP-2 promoter revealed that AP-1 site proximal to the transcriptional start site is required for 15(S)-HETE-induced MMP-2 expression, and Fra-1 and c-Jun are the essential components of AP-1 that bind to MMP-2 promoter in response to 15(S)-HETE. Hind limb ischemia led to an increase in MEK1 and JNK1 activation and Fra-1, c-Jun, and MMP-2 expression resulting in enhanced neovascularization and recovery of blood perfusion in wild-type mice as compared with 12/15-Lox(-/-) mice. Together, these results provide the first direct evidence for a role of 12/15-Lox-12/15(S)-HETE axis in the regulation of ischemia-induced angiogenesis.
Figures







Similar articles
-
12/15-Lipoxygenase gene knockout severely impairs ischemia-induced angiogenesis due to lack of Rac1 farnesylation.Blood. 2011 Nov 17;118(20):5701-12. doi: 10.1182/blood-2011-04-347468. Epub 2011 Aug 12. Blood. 2011. PMID: 21841162 Free PMC article.
-
15(S)-hydroxyeicosatetraenoic acid-induced angiogenesis requires Src-mediated Egr-1-dependent rapid induction of FGF-2 expression.Blood. 2010 Mar 11;115(10):2105-16. doi: 10.1182/blood-2009-09-241802. Epub 2010 Jan 6. Blood. 2010. PMID: 20053757 Free PMC article.
-
A novel role for activating transcription factor-2 in 15(S)-hydroxyeicosatetraenoic acid-induced angiogenesis.J Lipid Res. 2009 Mar;50(3):521-533. doi: 10.1194/jlr.M800388-JLR200. Epub 2008 Oct 10. J Lipid Res. 2009. PMID: 18849464 Free PMC article.
-
15(S)-HETE production in human retinal microvascular endothelial cells by hypoxia: Novel role for MEK1 in 15(S)-HETE induced angiogenesis.Invest Ophthalmol Vis Sci. 2007 Nov;48(11):4930-8. doi: 10.1167/iovs.07-0617. Invest Ophthalmol Vis Sci. 2007. PMID: 17962441
-
Vascular actions of 20-HETE.Prostaglandins Other Lipid Mediat. 2015 Jul;120:9-16. doi: 10.1016/j.prostaglandins.2015.03.002. Epub 2015 Mar 23. Prostaglandins Other Lipid Mediat. 2015. PMID: 25813407 Free PMC article. Review.
Cited by
-
PKCθ-JunB axis via upregulation of VEGFR3 expression mediates hypoxia-induced pathological retinal neovascularization.Cell Death Dis. 2020 May 7;11(5):325. doi: 10.1038/s41419-020-2522-0. Cell Death Dis. 2020. PMID: 32382040 Free PMC article.
-
MLK3 regulates FRA-1 and MMPs to drive invasion and transendothelial migration in triple-negative breast cancer cells.Oncogenesis. 2017 Jun 12;6(6):e345. doi: 10.1038/oncsis.2017.44. Oncogenesis. 2017. PMID: 28604765 Free PMC article.
-
AP-1 family transcription factors: a diverse family of proteins that regulate varied cellular activities in classical hodgkin lymphoma and ALK+ ALCL.Exp Hematol Oncol. 2021 Jan 7;10(1):4. doi: 10.1186/s40164-020-00197-9. Exp Hematol Oncol. 2021. PMID: 33413671 Free PMC article. Review.
-
Insidious role of nitric oxide in migration/invasion of colon cancer cells by upregulating MMP-2/9 via activation of cGMP-PKG-ERK signaling pathways.Clin Exp Metastasis. 2012 Jun;29(5):471-92. doi: 10.1007/s10585-012-9464-6. Epub 2012 Mar 15. Clin Exp Metastasis. 2012. PMID: 22419013
-
12/15-Lipoxygenase gene knockout severely impairs ischemia-induced angiogenesis due to lack of Rac1 farnesylation.Blood. 2011 Nov 17;118(20):5701-12. doi: 10.1182/blood-2011-04-347468. Epub 2011 Aug 12. Blood. 2011. PMID: 21841162 Free PMC article.
References
-
- Folkman J. (1995) Nat. Med. 1, 27–31 - PubMed
-
- Freedman S. B., Isner J. M. (2001) J. Mol. Cell Cardiol. 33, 379–393 - PubMed
-
- Mignatti P., Rifkin D. B. (1996) Enzyme Protein 49, 117–137 - PubMed
-
- Lauer-Fields J. L., Sritharan T., Stack M. S., Nagase H., Fields G. B. (2003) J. Biol. Chem. 278, 18140–18145 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

