Multiple roles for CCR2 during fracture healing
- PMID: 20354109
- PMCID: PMC2898536
- DOI: 10.1242/dmm.003186
Multiple roles for CCR2 during fracture healing
Abstract
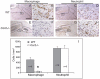
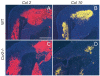
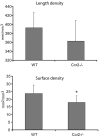
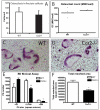
Bone injury induces an inflammatory response that involves neutrophils, macrophages and other inflammatory cells. The recruitment of inflammatory cells to sites of injury occurs in response to specific signaling pathways. The CC chemokine receptor type 2 (CCR2) is crucial for recruiting macrophages, as well as regulating osteoclast function. In this study, we examined fracture healing in Ccr2-/- mice. We first demonstrated that the expression of Ccr2 transcripts and the filtration of macrophages into fracture calluses were most robust during the early phases of fracture healing. We then determined that the number of macrophages at the fracture site was significantly lower in Ccr2-/- mice compared with wild-type controls at 3 days after injury. As a result, impaired vascularization, decreased formation of callus, and delayed maturation of cartilage were observed at 7 days after injury in mutant mice. At day 14, Ccr2-/- mice had less bone in their calluses. At day 21, Ccr2-/- mice had larger calluses and more bone compared with wild-type mice, suggesting a delayed remodeling. In addition, we examined the effect of Ccr2 mutation on osteoclasts. We found that a lack of Ccr2 did not affect the number of osteoclasts within fracture calluses at 21 days after injury. However, Ccr2-/- osteoclasts exhibited a decreased ability to resorb bone compared with wild-type cells, which could contribute to the delayed remodeling of fracture calluses observed in Ccr2-/- mice. Collectively, these results indicate that a deficiency of Ccr2 reduces the infiltration of macrophages and impairs the function of osteoclasts, leading to delayed fracture healing.
Figures

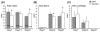
References
-
- Altman RD, Latta LL, Keer R, Renfree K, Hornicek FJ, Banovac K. (1995). Effect of nonsteroidal antiinflammatory drugs on fracture healing: a laboratory study in rats. J Orthop Trauma 9, 392–400 - PubMed
-
- Binder NB, Niederreiter B, Hoffmann O, Stange R, Pap T, Stulnig TM, Mack M, Erben RG, Smolen JS, Redlich K. (2009). Estrogen-dependent and C-C chemokine receptor-2-dependent pathways determine osteoclast behavior in osteoporosis. Nat Med. 15, 417–424 - PubMed
-
- Bonnarens F, Einhorn TA. (1984). Production of a standard closed fracture in laboratory animal bone. J Orthop Res. 2, 97–101 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

