Long-term follow-up of cervical disease in women screened by cytology and HPV testing: results from the HART study
- PMID: 20354519
- PMCID: PMC2865748
- DOI: 10.1038/sj.bjc.6605619
Long-term follow-up of cervical disease in women screened by cytology and HPV testing: results from the HART study
Abstract
Background: Several studies have shown that testing for high-risk human papillomavirus (HPV) types results in an improved sensitivity for CIN2+, compared with cytology, although with a somewhat lower specificity.
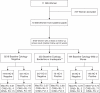
Methods: We obtained follow-up results, with at least one smear after participation in the HART study, which compared HPV testing (HC-II) with cytology as a primary screening modality.
Results: With a median follow-up of 6 years, 42 additional cases of CIN2+ were identified; women who were HPV positive at baseline were more likely to develop CIN2+ than those who were HPV negative (hazard ratio (HR) 17.2; 95% confidence interval (CI) (9.3-31.6)) and the risk increased with increasing viral load. Compared with HPV-negative women (relative light unit (RLU) <1), the HR (95% CI) was 5.4 (1.6, 18.2) for 1-10 RLU and 25.5 (13.6, 47.9) for RLU > or = 10. Positive cytology (borderline or worse compared with negative) was also predictive of developing CIN2, although to a lesser extent (HR 8.7; 95% CI (4.5-17.1)). Only one case of CIN3 and three cases of CIN2 were found in women who showed a positive cytology result but were HPV negative at baseline.
Conclusion: After 5 years of follow-up, CIN2+ occurred in 0.23% of women who were HPV negative at baseline compared with 0.48% of women who showed a negative cytology result, indicating a much longer low-risk interval for CIN2+ after HPV testing.
Figures


References
-
- Bray F, Loos AH, McCarron P, Weiderpass E, Arbyn M, Moller H, Hakama M, Parkin DM (2005) Trends in cervical squamous cell carcinoma incidence in 13 European countries: changing risk and the effects of screening. Cancer Epidemiol Biomarkers Prev 14: 677–686 - PubMed
-
- Bulkmans NW, Berkhof J, Rozendaal L, van Kemenade FJ, Boeke AJ, Bulk S, Voorhorst FJ, Verheijen RH, van Groningen K, Boon ME, Ruitinga W, van Ballegooijen M, Snijders PJ, Meijer CJ (2007) Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet 370: 1764–1772 - PubMed
-
- Clifford G, Franceschi S, Diaz M, Munoz N, Villa LL (2006) Chapter 3: HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine 24(Suppl 3): S3/26–S3/34 - PubMed
-
- Cuzick J, Clavel C, Petry KU, Meijer CJ, Hoyer H, Ratnam S, Szarewski A, Birembaut P, Kulasingam S, Sasieni P, Iftner T (2006) Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer 119: 1095–1101 - PubMed
-
- Cuzick J, Szarewski A, Cubie H, Hulman G, Kitchener H, Luesley D, McGoogan E, Menon U, Terry G, Edwards R, Brooks C, Desai M, Gie C, Ho L, Jacobs I, Pickles C, Sasieni P (2003) Management of women who test positive for high-risk types of human papillomavirus: the HART study. Lancet 362: 1871–1876 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

