An integrated model for enzyme catalysis emerges from studies of hydrogen tunneling
- PMID: 20354595
- PMCID: PMC2846846
- DOI: 10.1016/j.cplett.2009.01.038
An integrated model for enzyme catalysis emerges from studies of hydrogen tunneling
Abstract
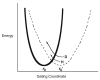
The origins of the enormous rate accelerations brought about by enzymes are discussed. The focus is on enzymatic C-H activation, which has been shown to take place via tunneling. Four enzyme systems illustrate the impact of site-specific mutagenesis, changes in temperature or changes in protein solvation on the tunneling properties. A model emerges in which conformational sampling is required to access a subset of protein conformers where the H-donor and acceptor undergo a close approach. The evidence for an inverse relationship between protein flexibility and active site compression is likely to extend to all classes of enzyme catalysts.
Figures

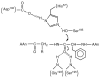

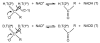
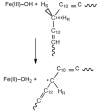
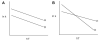

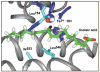
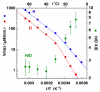
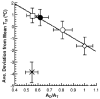
 , D and
, D and  , H) and of the H/D KIE (
, H) and of the H/D KIE ( ) for the ht-ADH.
) for the ht-ADH.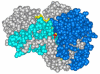


References
-
- Wolfenden R, Snider MJ. Acc. Chem. Res. 2001;34:938. - PubMed
-
- Dwyer MA, Looger LL, Hellinga HW. Science. 2008;319:569. - PubMed
-
- Jiang L, et al. Science. 2005;309:1868.
-
- Pauling L. Am. Sci. 1948;36:51. - PubMed
-
- Wolfenden R. In: Transition States of Biochemical Processes. Gandour RD, Schowen RL, editors. Plenum Press; New York and London: 1978. p. 558.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
