Intraneuronal beta-amyloid accumulation and synapse pathology in Alzheimer's disease
- PMID: 20354705
- PMCID: PMC3183823
- DOI: 10.1007/s00401-010-0679-9
Intraneuronal beta-amyloid accumulation and synapse pathology in Alzheimer's disease
Abstract
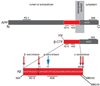
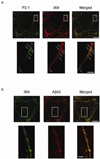
The aberrant accumulation of aggregated beta-amyloid peptides (Abeta) as plaques is a hallmark of Alzheimer's disease (AD) neuropathology and reduction of Abeta has become a leading direction of emerging experimental therapies for the disease. The mechanism(s) whereby Abeta is involved in the pathophysiology of the disease remain(s) poorly understood. Initially fibrils, and subsequently oligomers of extracellular Abeta have been viewed as the most important pathogenic form of Abeta in AD. More recently, the intraneuronal accumulation of Abeta has been described in the brain, although technical considerations and its relevance in AD have made this a controversial topic. Here, we review the emerging evidence linking intraneuronal Abeta accumulation to the development of synaptic pathology and plaques in AD, and discuss the implications of intraneuronal beta-amyloid for AD pathology, biology, diagnosis and therapy.
Conflict of interest statement
Figures


References
-
- Alafuzoff I, Pikkarainen M, Arzberger T, et al. Inter-laboratory comparison of neuropathological assessments of beta-amyloid protein: a study of the BrainNet Europe consortium. Acta Neuropathol. 2008;115:533–546. - PubMed
-
- Almeida CG, Tampellini D, Takahashi RH, et al. Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiol Dis. 2005;20:187–198. - PubMed
-
- Aoki M, Volkmann I, Tjernberg LO, Winblad B, Bogdanovic N. Amyloid betapeptide levels in laser capture microdissected cornu ammonis 1 pyramidal neurons of Alzheimer's brain. Neuroreport. 2008;19:1085–1089. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

