Macromolecular crowding induces polypeptide compaction and decreases folding cooperativity
- PMID: 20355290
- PMCID: PMC3050011
- DOI: 10.1039/b924236h
Macromolecular crowding induces polypeptide compaction and decreases folding cooperativity
Abstract
A cell's interior is comprised of macromolecules that can occupy up to 40% of its available volume. Such crowded environments can influence the stability of proteins and their rates of reaction. Using discrete molecular dynamics simulations, we investigate how both the size and number of neighboring crowding reagents affect the thermodynamic and folding properties of structurally diverse proteins. We find that crowding induces higher compaction of proteins. We also find that folding becomes less cooperative with the introduction of crowders into the system. The crowders may induce alternative non-native protein conformations, thus creating barriers for protein folding in highly crowded media.
Figures


 ) 0%, (
) 0%, ( ) 5%, (
) 5%, ( ) 13%, (-·-) 26%, (
) 13%, (-·-) 26%, ( ) 39%, (⋯) 52% fractional volume.
) 39%, (⋯) 52% fractional volume.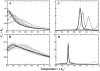
 ) 0%, (
) 0%, ( ) 5%, (
) 5%, ( ) 13%, (-·-) 26%, (
) 13%, (-·-) 26%, ( ) 39%, (⋯) 52% fractional volume. Gray regions indicate the estimated error for each curve.
) 39%, (⋯) 52% fractional volume. Gray regions indicate the estimated error for each curve.
 ) 0%, (
) 0%, ( ) 26%, (
) 26%, ( ) 39%, (-·-) 52% fractional volume. Gray regions indicate the estimated error for each curve. (B) Degree of cooperativity for Trp-cage as measured by the ratio κ2. Increasing concentrations of crowders lowers the two-state folding cooperativity.
) 39%, (-·-) 52% fractional volume. Gray regions indicate the estimated error for each curve. (B) Degree of cooperativity for Trp-cage as measured by the ratio κ2. Increasing concentrations of crowders lowers the two-state folding cooperativity.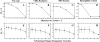

 ) 5 Å, (
) 5 Å, ( )10 Å, (
)10 Å, ( )15 Å, (-·-) 20 Å crowder diameter.
)15 Å, (-·-) 20 Å crowder diameter.
 ) 5 Å, (
) 5 Å, ( )10Å, (
)10Å, ( ) 15Å, (-·-) 20 Å crowder diameter. Gray regions indicate the estimated error associated with each curve. (B) Comparison of the degree of cooperativity for SH3 domain under various crowding conditions. The top X-axis corresponds to the open circles plotted and represent the set of simulations at constant fractional volume. For comparison we have plotted the degree of cooperativity as a function of crowder concentration at a constant diameter of 10 Å, measured on the bottom X-axis and denoted by closed squares. Dashed lines are plotted to aid in identifying the trends between the two sets. (C) Energy histogram plotted for SH3 domain under different crowding diameters at constant fractional volume. Two-state folding kinetics are represented by the folded state on the left and the unfolded state on the right. The diminishing and shifting peak representative of the unfolded state is indicative of effective compacting obtained by smaller crowders. Legend: (
) 15Å, (-·-) 20 Å crowder diameter. Gray regions indicate the estimated error associated with each curve. (B) Comparison of the degree of cooperativity for SH3 domain under various crowding conditions. The top X-axis corresponds to the open circles plotted and represent the set of simulations at constant fractional volume. For comparison we have plotted the degree of cooperativity as a function of crowder concentration at a constant diameter of 10 Å, measured on the bottom X-axis and denoted by closed squares. Dashed lines are plotted to aid in identifying the trends between the two sets. (C) Energy histogram plotted for SH3 domain under different crowding diameters at constant fractional volume. Two-state folding kinetics are represented by the folded state on the left and the unfolded state on the right. The diminishing and shifting peak representative of the unfolded state is indicative of effective compacting obtained by smaller crowders. Legend: ( ) No crowders, (
) No crowders, ( ) 20 Å, (
) 20 Å, ( )15 Å, (-·-) 5 Å.
)15 Å, (-·-) 5 Å.References
-
- Hall D, Minton AP. Biochim. Biophys. Acta, Proteins Proteomics. 2003;1649:127–139. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

