Conformational preferences of a 14-residue fibrillogenic peptide from acetylcholinesterase
- PMID: 20356043
- PMCID: PMC2860372
- DOI: 10.1021/bi1001807
Conformational preferences of a 14-residue fibrillogenic peptide from acetylcholinesterase
Abstract
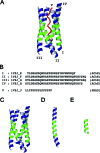
A 14-residue fragment from near the C-terminus of the enzyme acetylcholinesterase (AChE) is believed to have a neurotoxic/neurotrophic effect acting via an unknown pathway. While the peptide is alpha-helical in the full-length enzyme, the structure and association mechanism of the fragment are unknown. Using multiple molecular dynamics simulations, starting from a tetrameric complex of the association domain of AChE and systematically disassembled subsets that include the peptide fragment, we show that the fragment is incapable of retaining its helicity in solution. Extensive replica exchange Monte Carlo folding and unfolding simulations in implicit solvent with capped and uncapped termini failed to converge to any consistent cluster of structures, suggesting that the fragment remains largely unstructured in solution under the conditions considered. Furthermore, extended molecular dynamics simulations of two steric zipper models show that the peptide is likely to form a zipper with antiparallel sheets and that peptides with mutations known to prevent fibril formation likely do so by interfering with this packing. The results demonstrate how the local environment of a peptide can stabilize a particular conformation.
Figures



Similar articles
-
The intact human acetylcholinesterase C-terminal oligomerization domain is alpha-helical in situ and in isolation, but a shorter fragment forms beta-sheet-rich amyloid fibrils and protofibrillar oligomers.Biochemistry. 2003 Sep 16;42(36):10863-73. doi: 10.1021/bi034768i. Biochemistry. 2003. PMID: 12962511
-
Understanding amyloid fibril nucleation and aβ oligomer/drug interactions from computer simulations.Acc Chem Res. 2014 Feb 18;47(2):603-11. doi: 10.1021/ar4002075. Epub 2013 Dec 24. Acc Chem Res. 2014. PMID: 24368046 Review.
-
Modeling the alpha-helix to beta-hairpin transition mechanism and the formation of oligomeric aggregates of the fibrillogenic peptide Abeta(12-28): insights from all-atom molecular dynamics simulations.J Mol Graph Model. 2004 Dec;23(3):263-73. doi: 10.1016/j.jmgm.2004.07.004. J Mol Graph Model. 2004. PMID: 15530822
-
The C-terminal peptides of acetylcholinesterase: cellular trafficking, oligomerization and functional anchoring.Chem Biol Interact. 2005 Dec 15;157-158:3-14. doi: 10.1016/j.cbi.2005.10.002. Epub 2005 Oct 28. Chem Biol Interact. 2005. PMID: 16257397
-
Relationship between helix stability and binding affinities: molecular dynamics simulations of Bfl-1/A1-binding pro-apoptotic BH3 peptide helices in explicit solvent.J Biomol Struct Dyn. 2013;31(1):65-77. doi: 10.1080/07391102.2012.691363. Epub 2012 Jul 18. J Biomol Struct Dyn. 2013. PMID: 22803956
References
-
- Silman I.; Sussman J. L. (2005) Acetylcholinesterase: 'Classical’ and 'non-classical’ functions and pharmacology. Curr. Opin. Pharmacol. 5, 293–302. - PubMed
-
- Greenfield S. A.; Zimmermann M.; Bond C. E. (2008) Non-hydrolytic functions of acetylcholinesterase. The significance of C-terminal peptides. FEBS J. 275, 604–611. - PubMed
-
- Cottingham M. G.; Hollinshead M. S.; Vaux D. J. T. (2002) Amyloid fibril formation by a synthetic peptide from a region of human acetylcholinesterase that is homologous to the Alzheimer’s amyloid-β peptide. Biochemistry 41, 13539–13547. - PubMed
-
- Greenfield S. A.; Day T.; Mann E. O.; Bermudez I. (2004) A novel peptide modulates α7 nicotinic receptor responses: Implications for a possible trophic-toxic mechanism within the brain. J. Neurochem. 90, 325–331. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

