The tert-butylsulfinamide lynchpin in transition-metal-mediated multiscaffold library synthesis
- PMID: 20356070
- PMCID: PMC2869296
- DOI: 10.1021/ol100574y
The tert-butylsulfinamide lynchpin in transition-metal-mediated multiscaffold library synthesis
Abstract
A unified synthetic approach to diverse polycyclic scaffolds has been developed using transition-metal-mediated cycloaddition and cyclization reactions of enynes and diynes. The tert-butylsulfinamide group has been identified as a particularly versatile lynchpin in these reactions, with a reactivity profile uniquely suited for efficient, stereoselective substrate synthesis and downstream transformations. This approach provides 10 distinct, functionalized scaffold classes related to common core structures in alkaloid and terpenoid natural products.
Figures



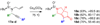
References
-
-
See Supporting Information for full details.
-
-
-
For reviews, see: Burke MD, Schreiber SL. Angew. Chem., Int. Ed. 2004;43:46–58. Nielsen TE, Schreiber SL. Angew. Chem., Int. Ed. 2008;47:48–56.
-
-
-
For selected key examples, see: Burke MD, Schreiber SL. Science. 2003;302:613–618. Taylor SJ, Taylor AM, Schreiber SL. Angew. Chem., Int. Ed. 2004;43:1681–1685. Oguri H, Schreiber SL. Org. Lett. 2005;7:47–50. Spiegel DA, Schroeder FC, Duvall JR, Schreiber SL. J. Am. Chem. Soc. 2006;128:14766–14767. Wyatt EE, Fergus S, Galloway WRJD, Bender A, Fox DJ, Plowright AT, Jessiman AS, Welch M, Spring DR. Chem. Commun. 2006:3296–3298.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

