Fluorescence lifetime measurements and biological imaging
- PMID: 20356094
- PMCID: PMC2924670
- DOI: 10.1021/cr900343z
Fluorescence lifetime measurements and biological imaging
Figures




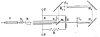
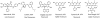
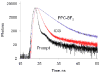
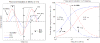









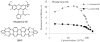
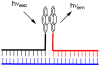

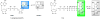
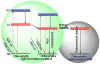





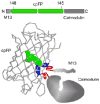
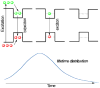
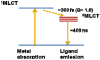






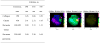
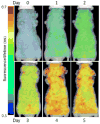
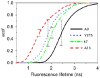
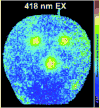

References
-
- McGown LB, Nithipatikom K. Appl Spectrosc Rev. 2000;35:353.
-
- Chen Y, Periasamy A. Microsc Res Tech. 2004;63:72. - PubMed
-
- Lakowicz JR. Principles of fluorescence spectroscopy. 3rd. Springer; New York: 2006.
-
- Becker W, Bergmann A, Biskup C. Microsc Res Tech. 2007;70:403. - PubMed
-
- Becker W, Bergmann A. In: Handbook of Biomedical Nonlinear Optical Microscopy. Master BR, So P, editors. Oxford University Press, USA; New York, NY: 2008.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

