Autotaxin expression and its connection with the TNF-alpha-NF-kappaB axis in human hepatocellular carcinoma
- PMID: 20356387
- PMCID: PMC2867819
- DOI: 10.1186/1476-4598-9-71
Autotaxin expression and its connection with the TNF-alpha-NF-kappaB axis in human hepatocellular carcinoma
Abstract
Background: Autotaxin (ATX) is an extracellular lysophospholipase D that generates lysophosphatidic acid (LPA) from lysophosphatidylcholine (LPC). Both ATX and LPA have been shown to be involved in many cancers. However, the functional role of ATX and the regulation of ATX expression in human hepatocellular carcinoma (HCC) remain elusive.
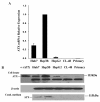
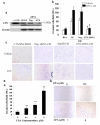
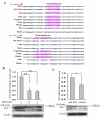
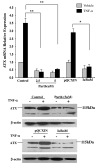
Results: In this study, ATX expression was evaluated in tissues from 38 human HCC and 10 normal control subjects. ATX was detected mainly in tumor cells within tissue sections and its over-expression in HCC was specifically correlated with inflammation and liver cirrhosis. In addition, ATX expression was examined in normal human hepatocytes and liver cancer cell lines. Hepatoma Hep3B and Huh7 cells displayed stronger ATX expression than hepatoblastoma HepG2 cells and normal hepatocytes did. Proinflammtory cytokine tumor necrosis factor alpha (TNF-alpha) promoted ATX expression and secretion selectively in Hep3B and Huh7 cells, which led to a corresponding increase in lysophospholipase-D activity. Moreover, we explored the mechanism governing the expression of ATX in hepatoma cells and established a critical role of nuclear factor-kappa B (NF-kappaB) in basal and TNF-alpha induced ATX expression. Further study showed that secreted enzymatically active ATX stimulated Hep3B cell invasion.
Conclusions: This report highlights for the first time the clinical and biological evidence for the involvement of ATX in human HCC. Our observation that links the TNF-alpha/NF-kappaB axis and the ATX-LPA signaling pathway suggests that ATX is likely playing an important role in inflammation related liver tumorigenesis.
Figures


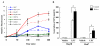
References
-
- Watanabe N, Ikeda H, Nakamura K, Ohkawa R, Kume Y, Tomiya T, Tejima K, Nishikawa T, Arai M, Yanase M. Plasma ysophosphatidic acid level and serum autotaxin activity are increased in liver injury in rats in relation to its severity. Life Sci. 2007;81:1009–1015. doi: 10.1016/j.lfs.2007.08.013. - DOI - PubMed
-
- Stracke ML, Krutzsch HC, Unsworth EJ, Arestad A, Cioce V, Schiffmann E, Liotta LA. Identification, purification, and partial sequence analysis of autotaxin, a novel motility-stimulating protein. J Biol Chem. 1992;267:2524–2529. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

