The initiation of embryonic-like collagen fibrillogenesis by adult human tendon fibroblasts when cultured under tension
- PMID: 20356622
- PMCID: PMC3485556
- DOI: 10.1016/j.biomaterials.2010.02.062
The initiation of embryonic-like collagen fibrillogenesis by adult human tendon fibroblasts when cultured under tension
Abstract
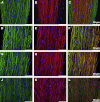
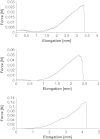
Tendon fibroblasts synthesize collagen and form fibrils during embryonic development, but to what extent mature fibroblasts are able to recapitulate embryonic development and develop normal tendon structure is unknown. The present study examined the capability of mature human tendon fibroblasts to initiate collagen fibrillogenesis when cultured in fixed-length fibrin gels. Fibroblasts were dissected from semitendinosus and gracilis tendons from healthy humans and cultured in 3D linear fibrin gels. The fibroblasts synthesized an extracellular matrix of parallel collagen fibrils that were aligned along the axis of tension. The fibrils had a homogeneous narrow diameter that was similar to collagen fibrils occurring in embryonic tendon. Immunostaining showed colocalization of collagen type I with collagen III, XII and XIV. A fibronectin network was formed in parallel with the collagen, and fibroblasts stained positive for integrin alpha(5). Finally, the presence of cell extensions into the extracellular space with membrane-enclosed fibrils in fibripositors indicated characteristics of embryonic tendon. We conclude that mature human tendon fibroblasts retain an intrinsic capability to perform collagen fibrillogenesis similar to that of developing tendon, which implies that the hormonal/mechanical milieu, rather than intrinsic cellular function, inhibits regenerative potential in mature tendon.
(c) 2010 Elsevier Ltd. All rights reserved.
Figures






References
-
- Kastelic J., Galeski A., Baer E. The multicomposite structure of tendon. Connect Tissue Res. 1978;6(1):11–23. - PubMed
-
- Humphries S.M., Lu Y., Canty E.G., Kadler K.E. Active negative control of collagen fibrillogenesis in vivo. Intracellular cleavage of the type I procollagen propeptides in tendon fibroblasts without intracellular fibrils. J Biol Chem. 2008;283(18):12129–12135. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

