Enteric reovirus infection stimulates peanut-specific IgG2a responses in a mouse food allergy model
- PMID: 20356650
- PMCID: PMC2908721
- DOI: 10.1016/j.imbio.2010.02.004
Enteric reovirus infection stimulates peanut-specific IgG2a responses in a mouse food allergy model
Abstract
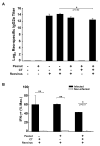
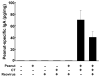
IgE-mediated food allergies are an important cause of life-threatening hypersensitivity reactions. Orally administered peanut antigens mixed with the mucosal adjuvant cholera toxin (CT) induce a strong peanut extract (PE)-specific serum IgE response that is correlated with T-helper type 1 (Th1) and type 2 (Th2)-like T-cell responses. This study was conducted to determine if respiratory enteric orphan virus (reovirus), a non-pathogenic virus that induces robust Th1-mediated mucosal and systemic responses could modulate induction of PE-specific allergic responses when co-administered with PE. Young mice were orally exposed to PE mixed with CT, reovirus, or both CT and reovirus. As expected, CT promoted PE-specific serum IgE, IgG1, and IgG2a and intestinal IgA production as well as splenic Th1- and Th2-associated cytokine recall responses. Reovirus did not alter PE-specific serum IgE and IgG1 levels, but substantially increased the PE-specific IgG2a response when co-administered with PE with or without CT. Additionally, reovirus significantly decreased the percentage of the Peyer's patch CD8+ T-cells and Foxp3+CD4+ T-regulatory cells when co-administered with PE. These results demonstrate that an acute mucosal reovirus infection and subsequent Th1 immune response is capable of modulating the Th1/Th2 controlled humoral response to PE. The reovirus-mediated increase in the PE-specific IgG2a antibody response may have therapeutic implications as increased levels of non-allergenic PE-specific IgG2a could block PE antigens from binding to IgE-sensitized mast cells.
Copyright © 2010 Elsevier GmbH. All rights reserved.
Figures



Similar articles
-
Activation of the aryl hydrocarbon receptor suppresses sensitization in a mouse peanut allergy model.Toxicol Sci. 2011 Oct;123(2):491-500. doi: 10.1093/toxsci/kfr175. Epub 2011 Jul 29. Toxicol Sci. 2011. PMID: 21804081
-
Spectrum of gut immunologic reactions: selective induction of distinct responses to Vibrio cholerae WO7 and its toxin.Microbiol Immunol. 2000;44(11):931-40. doi: 10.1111/j.1348-0421.2000.tb02585.x. Microbiol Immunol. 2000. PMID: 11145274
-
Modulation of mucosal immunity in a murine model of food-induced intestinal inflammation.Clin Exp Allergy. 2008 Feb;38(2):338-49. doi: 10.1111/j.1365-2222.2007.02866.x. Epub 2007 Nov 13. Clin Exp Allergy. 2008. PMID: 18005184
-
The common mucosal immune system: from basic principles to enteric vaccines with relevance for the female reproductive tract.Reprod Fertil Dev. 1994;6(3):369-79. doi: 10.1071/rd9940369. Reprod Fertil Dev. 1994. PMID: 7831485 Review.
-
Experimental intestinal reovirus infection of mice: what we know, what we need to know.Immunol Res. 2005;33(3):257-65. doi: 10.1385/IR:33:3:257. Immunol Res. 2005. PMID: 16462002 Free PMC article. Review.
Cited by
-
AllerML: markup language for allergens.Regul Toxicol Pharmacol. 2011 Jun;60(1):151-60. doi: 10.1016/j.yrtph.2011.03.006. Epub 2011 Mar 21. Regul Toxicol Pharmacol. 2011. PMID: 21420460 Free PMC article.
-
Cross-reactive MHC class I T cell epitopes may dictate heterologous immune responses between respiratory viruses and food allergens.Sci Rep. 2023 Sep 8;13(1):14874. doi: 10.1038/s41598-023-41187-1. Sci Rep. 2023. PMID: 37684288 Free PMC article.
-
Reovirus type-2 infection in newborn DBA/1J mice reduces the development of late allergic asthma.Int J Exp Pathol. 2012 Jun;93(3):234-42. doi: 10.1111/j.1365-2613.2012.00816.x. Int J Exp Pathol. 2012. PMID: 22583134 Free PMC article.
-
Development of a reverse transcription loop mediated isothermal amplification assay for the detection of Mouse reovirus type 3 in laboratory mice.Sci Rep. 2021 Feb 10;11(1):3508. doi: 10.1038/s41598-021-83034-1. Sci Rep. 2021. PMID: 33568687 Free PMC article.
-
Peyer's patches and mesenteric lymph nodes cooperatively promote enteropathy in a mouse model of food allergy.PLoS One. 2014 Oct 7;9(10):e107492. doi: 10.1371/journal.pone.0107492. eCollection 2014. PLoS One. 2014. PMID: 25290461 Free PMC article.
References
-
- Adel-Patient K, Ah-Leung S, Bernard H, Durieux-Alexandrenne C, Creminon C, Wal JM. Oral sensitization to peanut is highly enhanced by application of peanut extracts to intact skin, but is prevented when CpG and cholera toxin are added. Int. Arch. Allergy Immunol. 2007;143:10–20. - PubMed
-
- Bashir ME, Louie S, Shi HN, Nagler-Anderson C. Toll-like receptor 4 signaling by intestinal microbes influences susceptibility to food allergy. J Immunol. 2004;172:6978–6987. - PubMed
-
- Burks AW. Peanut allergy. Lancet. 2008;371:1538–1546. - PubMed
-
- Calvani M, Alessandri C, Paolone G, Rosengard L, Di Caro A, De Franco D. Correlation between Epstein Barr virus antibodies, serum IgE and atopic disease. Pediatr. Allergy Immunol. 1997;8:91–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

