Whole tumor antigen vaccines
- PMID: 20356763
- PMCID: PMC3119500
- DOI: 10.1016/j.smim.2010.02.004
Whole tumor antigen vaccines
Abstract
Although cancer vaccines with defined antigens are commonly used, the use of whole tumor cell preparations in tumor immunotherapy is a very promising approach and can obviate some important limitations in vaccine development. Whole tumor cells are a good source of TAAs and can induce simultaneous CTLs and CD4(+) T helper cell activation. We review current approaches to prepare whole tumor cell vaccines, including traditional methods of freeze-thaw lysates, tumor cells treated with ultraviolet irradiation, and RNA electroporation, along with more recent methods to increase tumor cell immunogenicity with HOCl oxidation or infection with replication-incompetent herpes simplex virus.
Copyright 2010. Published by Elsevier Ltd.
Figures
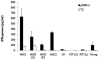
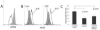
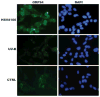

Similar articles
-
A dendritic cell vaccine pulsed with autologous hypochlorous acid-oxidized ovarian cancer lysate primes effective broad antitumor immunity: from bench to bedside.Clin Cancer Res. 2013 Sep 1;19(17):4801-15. doi: 10.1158/1078-0432.CCR-13-1185. Epub 2013 Jul 9. Clin Cancer Res. 2013. PMID: 23838316 Free PMC article.
-
Immunogenic potential of three transmissible venereal tumor cell lysates to prime canine-dendritic cells for cancer immunotherapy.Res Vet Sci. 2018 Dec;121:23-30. doi: 10.1016/j.rvsc.2018.10.001. Epub 2018 Oct 5. Res Vet Sci. 2018. PMID: 30316013
-
Clinically feasible approaches to potentiating cancer cell-based immunotherapies.Hum Vaccin Immunother. 2015;11(4):851-69. doi: 10.1080/21645515.2015.1009814. Hum Vaccin Immunother. 2015. PMID: 25933181 Free PMC article. Review.
-
Adjuvants for enhancing the immunogenicity of whole tumor cell vaccines.Int Rev Immunol. 2011 Apr-Jun;30(2-3):150-82. doi: 10.3109/08830185.2011.572210. Int Rev Immunol. 2011. PMID: 21557641 Review.
-
The present status and future prospects of peptide-based cancer vaccines.Int Immunol. 2016 Jul;28(7):319-28. doi: 10.1093/intimm/dxw027. Epub 2016 May 28. Int Immunol. 2016. PMID: 27235694 Review.
Cited by
-
Antigenic Essence: Upgrade of Cellular Cancer Vaccines.Cancers (Basel). 2021 Feb 12;13(4):774. doi: 10.3390/cancers13040774. Cancers (Basel). 2021. PMID: 33673325 Free PMC article. Review.
-
Capability of Human Dendritic Cells Pulsed with Autologous Induced Pluripotent Stem Cell Lysate to Induce Cytotoxic T Lymphocytes against HLA-A33-Matched Cancer Cells.Int J Mol Sci. 2022 Oct 27;23(21):12992. doi: 10.3390/ijms232112992. Int J Mol Sci. 2022. PMID: 36361783 Free PMC article.
-
Modulation of the Tumor Microenvironment by CXCR4 Antagonist-Armed Viral Oncotherapy Enhances the Antitumor Efficacy of Dendritic Cell Vaccines against Neuroblastoma in Syngeneic Mice.Viruses. 2018 Aug 26;10(9):455. doi: 10.3390/v10090455. Viruses. 2018. PMID: 30149659 Free PMC article.
-
A Comprehensive Preclinical Model Evaluating the Recombinant PRAME Antigen Combined With the AS15 Immunostimulant to Fight Against PRAME-expressing Tumors.J Immunother. 2015 Oct;38(8):311-20. doi: 10.1097/CJI.0000000000000095. J Immunother. 2015. PMID: 26325375 Free PMC article.
-
Transforming Cancer Nanotechnology Data Analysis and User Experience. Part I: Current Challenges and Solutions Provided by caNanoLab.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2025 Jul-Aug;17(4):e70030. doi: 10.1002/wnan.70030. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2025. PMID: 40826521 Free PMC article. Review.
References
-
- Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–13. - PubMed
-
- Ioannides CG, Platsoucas CD, Rashed S, et al. Tumor cytolysis by lymphocytes infiltrating ovarian malignant ascites. Cancer Res. 1991;51:4257–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

