The mitochondrial 2-oxoglutarate carrier is part of a metabolic pathway that mediates glucose- and glutamine-stimulated insulin secretion
- PMID: 20356834
- PMCID: PMC2878081
- DOI: 10.1074/jbc.M109.092593
The mitochondrial 2-oxoglutarate carrier is part of a metabolic pathway that mediates glucose- and glutamine-stimulated insulin secretion
Abstract
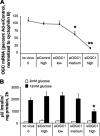
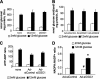
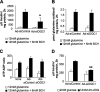
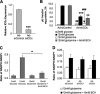
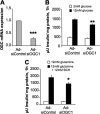
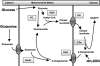
Glucose-stimulated insulin secretion from pancreatic islet beta-cells is dependent in part on pyruvate cycling through the pyruvate/isocitrate pathway, which generates cytosolic alpha-ketoglutarate, also known as 2-oxoglutarate (2OG). Here, we have investigated if mitochondrial transport of 2OG through the 2-oxoglutarate carrier (OGC) participates in control of nutrient-stimulated insulin secretion. Suppression of OGC in clonal pancreatic beta-cells (832/13 cells) and isolated rat islets by adenovirus-mediated delivery of small interfering RNA significantly decreased glucose-stimulated insulin secretion. OGC suppression also reduced insulin secretion in response to glutamine plus the glutamate dehydrogenase activator 2-amino-2-norbornane carboxylic acid. Nutrient-stimulated increases in glucose usage, glucose oxidation, glutamine oxidation, or ATP:ADP ratio were not affected by OGC knockdown, whereas suppression of OGC resulted in a significant decrease in the NADPH:NADP(+) ratio during stimulation with glucose but not glutamine + 2-amino-2-norbornane carboxylic acid. Finally, OGC suppression reduced insulin secretion in response to a membrane-permeant 2OG analog, dimethyl-2OG. These data reveal that the OGC is part of a mechanism of fuel-stimulated insulin secretion that is common to glucose, amino acid, and organic acid secretagogues, involving flux through the pyruvate/isocitrate cycling pathway. Although the components of this pathway must remain intact for appropriate stimulus-secretion coupling, production of NADPH does not appear to be the universal second messenger signal generated by these reactions.
Figures

Similar articles
-
The dicarboxylate carrier plays a role in mitochondrial malate transport and in the regulation of glucose-stimulated insulin secretion from rat pancreatic beta cells.Diabetologia. 2011 Jan;54(1):135-45. doi: 10.1007/s00125-010-1923-5. Epub 2010 Oct 15. Diabetologia. 2011. PMID: 20949348
-
Cytosolic and mitochondrial malic enzyme isoforms differentially control insulin secretion.J Biol Chem. 2007 Jan 5;282(1):200-7. doi: 10.1074/jbc.M602954200. Epub 2006 Nov 13. J Biol Chem. 2007. PMID: 17102138
-
Export of metabolites from pancreatic islet mitochondria as a means to study anaplerosis in insulin secretion.Metabolism. 2003 Aug;52(8):993-8. doi: 10.1016/s0026-0495(03)00149-5. Metabolism. 2003. PMID: 12898463
-
Overcoming the spatial barriers of the stimulus secretion cascade in pancreatic β-cells.Islets. 2012 Jan-Feb;4(1):1-116. doi: 10.4161/isl.18338. Epub 2011 Dec 6. Islets. 2012. PMID: 22143007 Review.
-
Contribution of Mitochondria to Insulin Secretion by Various Secretagogues.Antioxid Redox Signal. 2022 May;36(13-15):920-952. doi: 10.1089/ars.2021.0113. Epub 2021 Aug 24. Antioxid Redox Signal. 2022. PMID: 34180254 Free PMC article. Review.
Cited by
-
Control of voltage-gated potassium channel Kv2.2 expression by pyruvate-isocitrate cycling regulates glucose-stimulated insulin secretion.J Biol Chem. 2013 Aug 9;288(32):23128-40. doi: 10.1074/jbc.M113.491654. Epub 2013 Jun 20. J Biol Chem. 2013. PMID: 23788641 Free PMC article.
-
Mechanisms of β-cell functional adaptation to changes in workload.Diabetes Obes Metab. 2016 Sep;18 Suppl 1(Suppl 1):78-86. doi: 10.1111/dom.12729. Diabetes Obes Metab. 2016. PMID: 27615135 Free PMC article. Review.
-
Inhibition of the malate-aspartate shuttle in mouse pancreatic islets abolishes glucagon secretion without affecting insulin secretion.Biochem J. 2015 May 15;468(1):49-63. doi: 10.1042/BJ20140697. Biochem J. 2015. PMID: 25731850 Free PMC article.
-
The new biology of diabetes.Diabetologia. 2015 Nov;58(11):2459-68. doi: 10.1007/s00125-015-3722-5. Epub 2015 Aug 7. Diabetologia. 2015. PMID: 26248647 Free PMC article. Review.
-
Changes in mitochondrial carriers exhibit stress-specific signatures in INS-1Eβ-cells exposed to glucose versus fatty acids.PLoS One. 2013 Dec 12;8(12):e82364. doi: 10.1371/journal.pone.0082364. eCollection 2013. PLoS One. 2013. PMID: 24349266 Free PMC article.
References
-
- Ashcroft F. M., Harrison D. E., Ashcroft S. J. (1984) Nature 312, 446–448 - PubMed
-
- Bryan J., Crane A., Vila-Carriles W. H., Babenko A. P., Aguilar-Bryan L. (2005) Curr. Pharm. Des. 11, 2699–2716 - PubMed
-
- Newgard C. B., Matschinsky F. M. (2001) in Handbook of Physiology (Jefferson L. S., Cherrington A. eds) pp. 125–152, Oxford University Press, Oxford
-
- Henquin J. C. (2000) Diabetes 49, 1751–1760 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

