Computational model of cell positioning: directed and collective migration in the intestinal crypt epithelium
- PMID: 20356873
- PMCID: PMC2943877
- DOI: 10.1098/rsif.2010.0018.focus
Computational model of cell positioning: directed and collective migration in the intestinal crypt epithelium
Abstract
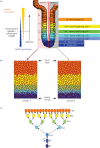
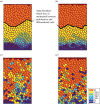
The epithelium of the intestinal crypt is a dynamic tissue undergoing constant regeneration through cell growth, cell division, cell differentiation and apoptosis. How the epithelial cells maintain correct positioning and how they migrate in a directed and collective fashion are still not well understood. In this paper, we developed a computational model to elucidate these processes. We show that differential adhesion between epithelial cells, caused by the differential activation of EphB receptors and ephrinB ligands along the crypt axis, is necessary to regulate cell positioning. Differential cell adhesion has been proposed previously to guide cell movement and cause cell sorting in biological tissues. The proliferative cells and the differentiated post-mitotic cells do not intermingle as long as differential adhesion is maintained. We also show that, without differential adhesion, Paneth cells are randomly distributed throughout the intestinal crypt. In addition, our model suggests that, with differential adhesion, cells migrate more rapidly as they approach the top of the intestinal crypt. Finally, by calculating the spatial correlation function of the cell velocities, we observe that differential adhesion results in the differentiated epithelial cells moving in a coordinated manner, where correlated velocities are maintained at large distances, suggesting that differential adhesion regulates coordinated migration of cells in tissues.
Figures


Similar articles
-
Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB.Cell. 2002 Oct 18;111(2):251-63. doi: 10.1016/s0092-8674(02)01015-2. Cell. 2002. PMID: 12408869
-
Control of cell adhesion and compartmentalization in the intestinal epithelium.Exp Cell Res. 2011 Nov 15;317(19):2695-701. doi: 10.1016/j.yexcr.2011.07.019. Epub 2011 Jul 29. Exp Cell Res. 2011. PMID: 21820431 Review.
-
Foxl1-deficient mice exhibit aberrant epithelial cell positioning resulting from dysregulated EphB/EphrinB expression in the small intestine.Am J Physiol Gastrointest Liver Physiol. 2006 Jul;291(1):G163-70. doi: 10.1152/ajpgi.00019.2006. Epub 2006 Feb 9. Am J Physiol Gastrointest Liver Physiol. 2006. PMID: 16469829
-
Computational models reveal a passive mechanism for cell migration in the crypt.PLoS One. 2013 Nov 18;8(11):e80516. doi: 10.1371/journal.pone.0080516. eCollection 2013. PLoS One. 2013. PMID: 24260407 Free PMC article.
-
Eph-ephrin signalling in adult tissues and cancer.Curr Opin Cell Biol. 2008 Apr;20(2):194-200. doi: 10.1016/j.ceb.2008.01.011. Epub 2008 Mar 18. Curr Opin Cell Biol. 2008. PMID: 18353626 Review.
Cited by
-
CoGNaC: A Chaste Plugin for the Multiscale Simulation of Gene Regulatory Networks Driving the Spatial Dynamics of Tissues and Cancer.Cancer Inform. 2015 Sep 1;14(Suppl 4):53-65. doi: 10.4137/CIN.S19965. eCollection 2015. Cancer Inform. 2015. PMID: 26380549 Free PMC article.
-
Epithelial and mesenchymal phenotypic switchings modulate cell motility in metastasis.Front Biosci (Landmark Ed). 2011 Jan 1;16(3):815-37. doi: 10.2741/3722. Front Biosci (Landmark Ed). 2011. PMID: 21196205 Free PMC article. Review.
-
Multiscale Model of Colorectal Cancer Using the Cellular Potts Framework.Cancer Inform. 2015 Oct 4;14(Suppl 4):83-93. doi: 10.4137/CIN.S19332. eCollection 2015. Cancer Inform. 2015. PMID: 26461973 Free PMC article.
-
A calibrated agent-based computer model of stochastic cell dynamics in normal human colon crypts useful for in silico experiments.Theor Biol Med Model. 2013 Nov 18;10:66. doi: 10.1186/1742-4682-10-66. Theor Biol Med Model. 2013. PMID: 24245614 Free PMC article.
-
Cell-Cell Adhesion and Cortical Actin Bending Govern Cell Elongation on Negatively Curved Substrates.Biophys J. 2018 Apr 10;114(7):1707-1717. doi: 10.1016/j.bpj.2018.02.027. Biophys J. 2018. PMID: 29642039 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

