Highly persistent and effective prime/boost regimens against tuberculosis that use a multivalent modified vaccine virus Ankara-based tuberculosis vaccine with interleukin-15 as a molecular adjuvant
- PMID: 20357059
- PMCID: PMC2863387
- DOI: 10.1128/CVI.00006-10
Highly persistent and effective prime/boost regimens against tuberculosis that use a multivalent modified vaccine virus Ankara-based tuberculosis vaccine with interleukin-15 as a molecular adjuvant
Abstract
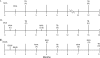
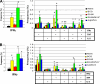
Novel immunization strategies are needed to enhance the global control of tuberculosis (TB). In this study, we assessed the immunizing activity of a recombinant modified vaccinia Ankara (MVA) construct (MVA/IL-15/5Mtb) which overexpresses five Mycobacterium tuberculosis antigens (antigen 85A, antigen 85B, ESAT6, HSP60, and Mtb39), as well as the molecular adjuvant interleukin-15 (IL-15). Homologous prime/boost studies showed that the MVA/IL-15/5Mtb vaccine induced moderate but highly persistent protective immune responses for at least 16 months after the initial vaccination and that the interval between the prime and boost did not significantly alter vaccine-induced antituberculosis protective immunity. At 16 months, when the Mycobacterium bovis BCG and MVA/IL-15/5Mtb vaccine-induced protection was essentially equivalent, the protective responses after a tuberculous challenge were associated with elevated levels of gamma interferon (IFN-gamma), IL-17F, Cxcl9, and Cxcl10. To amplify the immunizing potential of the MVA/IL-15/5Mtb vaccine, a heterologous prime/boost regimen was tested using an ESAT6-antigen 85B (E6-85) fusion protein formulated in dimethyldiotacylammonium bromide/monophosphoryl lipid A (DDA/MPL) adjuvant as the priming vaccine and the MVA/IL-15/5Mtb recombinant virus as the boosting agent. When MVA/IL-15/5Mtb vaccine boosting was done at 2 or 6 months following the final fusion protein injections, the prime/boost regimen evoked protective responses against an aerogenic M. tuberculosis challenge which was equivalent to that induced by BCG immunization. Long-term memory after immunization with the E6-85-MVA/IL-15/5Mtb combination regimen was associated with the induction of monofunctional CD4 and CD8 IFN-gamma-producing T cells and multifunctional CD4 and CD8 T cells expressing IFN-gamma/tumor necrosis factor alpha (TNF-alpha), TNF-alpha/IL-2, and IFN-gamma/TNF-alpha/IL-2. In contrast, BCG-induced protection was characterized by fewer CD4 and CD8 monofunctional T cells expressing IFN-gamma and only IFN-gamma/TNF-alpha and IFN-gamma/TNF-alpha/IL-2 expressing multifunctional T (MFT) cells. Taken together, these results suggest that a heterologous prime/boost protocol using an MVA-based tuberculosis vaccines to boost after priming with TB protein/adjuvant preparations should be considered when designing long-lived TB immunization strategies.
Figures
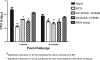
Similar articles
-
Performance of Homologous and Heterologous Prime-Boost Immunization Regimens of Recombinant Adenovirus and Modified Vaccinia Virus Ankara Expressing an Ag85B-TB10.4 Fusion Protein against Mycobacterium tuberculosis.J Microbiol Biotechnol. 2018 Jun 28;28(6):1022-1029. doi: 10.4014/jmb.1712.12064. J Microbiol Biotechnol. 2018. PMID: 29847865
-
Comparing adjuvanted H28 and modified vaccinia virus ankara expressingH28 in a mouse and a non-human primate tuberculosis model.PLoS One. 2013 Aug 19;8(8):e72185. doi: 10.1371/journal.pone.0072185. eCollection 2013. PLoS One. 2013. PMID: 23977248 Free PMC article.
-
CD4 and CD8 T cell responses to the M. tuberculosis Ag85B-TB10.4 promoted by adjuvanted subunit, adenovector or heterologous prime boost vaccination.PLoS One. 2009;4(4):e5139. doi: 10.1371/journal.pone.0005139. Epub 2009 Apr 9. PLoS One. 2009. PMID: 19357780 Free PMC article.
-
Immunogenic potential of latency associated antigens against Mycobacterium tuberculosis.Vaccine. 2014 Feb 3;32(6):712-6. doi: 10.1016/j.vaccine.2013.11.065. Epub 2013 Dec 2. Vaccine. 2014. PMID: 24300592 Review.
-
[Novel vaccines against M. tuberculosis].Kekkaku. 2006 Dec;81(12):745-51. Kekkaku. 2006. PMID: 17240920 Review. Japanese.
Cited by
-
Promising Cytokine Adjuvants for Enhancing Tuberculosis Vaccine Immunity.Vaccines (Basel). 2024 Apr 29;12(5):477. doi: 10.3390/vaccines12050477. Vaccines (Basel). 2024. PMID: 38793728 Free PMC article. Review.
-
Schistosoma mansoni antigen Sm-p80: Prophylactic efficacy of a vaccine formulated in human approved plasmid vector and adjuvant (VR 1020 and alum).Acta Trop. 2011 May;118(2):142-51. doi: 10.1016/j.actatropica.2011.01.010. Epub 2011 Feb 18. Acta Trop. 2011. PMID: 21334302 Free PMC article.
-
Human cord blood γδ T cells expressing public Vγ2 chains dominate the response to bisphosphonate plus interleukin-15.Immunology. 2013 Apr;138(4):346-60. doi: 10.1111/imm.12039. Immunology. 2013. PMID: 23181340 Free PMC article.
-
Functional genomic analysis of the 68-1 RhCMV-Mycobacteria tuberculosis vaccine reveals an IL-15 response signature that is conserved with vector attenuation.Front Immunol. 2024 Oct 15;15:1460344. doi: 10.3389/fimmu.2024.1460344. eCollection 2024. Front Immunol. 2024. PMID: 39474415 Free PMC article.
-
Short or Long Interval between Priming and Boosting: Does It Impact on the Vaccine Immunogenicity?Vaccines (Basel). 2021 Mar 20;9(3):289. doi: 10.3390/vaccines9030289. Vaccines (Basel). 2021. PMID: 33804604 Free PMC article.
References
-
- Beveridge, N. E., D. A. Price, J. P. Casazza, A. A. Pathan, C. R. Sander, T. E. Asher, D. R. Ambrozak, M. L. Precopio, P. Scheinberg, N. C. Alder, M. Roederer, R. A. Koup, D. C. Douek, A. V. Hill, and H. McShane. 2007. Immunisation with BCG and recombinant MVA85A induces long-lasting, polyfunctional Mycobacterium tuberculosis-specific CD4+ memory T lymphocyte populations. Eur. J. Immunol. 37:3089-3100. - PMC - PubMed
-
- Brewer, T. F. 2000. Preventing tuberculosis with bacillus Calmette-Guérin vaccine: a meta-analysis of the literature. Clin. Infect. Dis. 31:S64-S67. - PubMed
-
- Brice, G. T., C. Dobaño, M. Sedegah, M. Stefaniak, N. L. Graber, J. J. Campo, D. J. Carucci, and D. L. Doolan. 2007. Extended immunization intervals enhance the immunogenicity and protective efficacy of plasmid DNA vaccines. Microbes Infect. 9:1439-1446. - PubMed
-
- Colditz, G. A., T. F. Brewer, C. S. Berkey, M. E. Wilson, E. Burdick, H. V. Fineberg, and F. Mosteller. 1994. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 271:698-702. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

