Murine coronavirus delays expression of a subset of interferon-stimulated genes
- PMID: 20357099
- PMCID: PMC2876584
- DOI: 10.1128/JVI.00211-10
Murine coronavirus delays expression of a subset of interferon-stimulated genes
Abstract
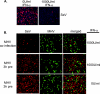
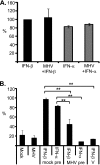
The importance of the type I interferon (IFN-I) system in limiting coronavirus replication and dissemination has been unequivocally demonstrated by rapid lethality following infection of mice lacking the alpha/beta IFN (IFN-alpha/beta) receptor with mouse hepatitis virus (MHV), a murine coronavirus. Interestingly, MHV has a cell-type-dependent ability to resist the antiviral effects of IFN-alpha/beta. In primary bone-marrow-derived macrophages and mouse embryonic fibroblasts, MHV replication was significantly reduced by the IFN-alpha/beta-induced antiviral state, whereas IFN treatment of cell lines (L2 and 293T) has only minor effects on replication (K. M. Rose and S. R. Weiss, Viruses 1:689-712, 2009). Replication of other RNA viruses, including Theiler's murine encephalitis virus (TMEV), vesicular stomatitis virus (VSV), Sindbis virus, Newcastle disease virus (NDV), and Sendai virus (SeV), was significantly inhibited in L2 cells treated with IFN-alpha/beta, and MHV had the ability to rescue only SeV replication. We present evidence that MHV infection can delay interferon-stimulated gene (ISG) induction mediated by both SeV and IFN-beta but only when MHV infection precedes SeV or IFN-beta exposure. Curiously, we observed no block in the well-defined IFN-beta signaling pathway that leads to STAT1-STAT2 phosphorylation and translocation to the nucleus in cultures infected with MHV. This observation suggests that MHV must inhibit an alternative IFN-induced pathway that is essential for early induction of ISGs. The ability of MHV to delay SeV-mediated ISG production may partially involve limiting the ability of IFN regulatory factor 3 (IRF-3) to function as a transcription factor. Transcription from an IRF-3-responsive promoter was partially inhibited by MHV; however, IRF-3 was transported to the nucleus and bound DNA in MHV-infected cells superinfected with SeV.
Figures
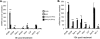


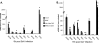

References
-
- Andersen, J., S. VanScoy, T. F. Cheng, D. Gomez, and N. C. Reich. 2008. IRF-3-dependent and augmented target genes during viral infection. Genes Immun. 9:168-175. - PubMed
-
- Andrejeva, J., D. F. Young, S. Goodbourn, and R. E. Randall. 2002. Degradation of STAT1 and STAT2 by the V proteins of simian virus 5 and human parainfluenza virus type 2, respectively: consequences for virus replication in the presence of alpha/beta and gamma interferons. J. Virol. 76:2159-2167. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

