Different relationship of N- and P/Q-type Ca2+ channels to channel-interacting slots in controlling neurotransmission at cultured hippocampal synapses
- PMID: 20357104
- PMCID: PMC3842455
- DOI: 10.1523/JNEUROSCI.5161-09.2010
Different relationship of N- and P/Q-type Ca2+ channels to channel-interacting slots in controlling neurotransmission at cultured hippocampal synapses
Abstract
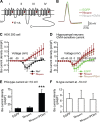
Synaptic transmission at CNS synapses is often mediated by joint actions of multiple Ca(2+) channel subtypes, most prominently, P/Q- and N-type. We have proposed that P/Q-type Ca(2+) channels saturate type-preferring slots at presynaptic terminals, which impose a ceiling on the synaptic efficacy of the channels. To test for analogous interactions for presynaptic N-type Ca(2+) channels, we overexpressed their pore-forming Ca(V)2.2 subunit in cultured mouse hippocampal neurons, recorded excitatory synaptic transmission from transfected cells, and dissected the contributions of N-, P/Q-, and R-type channels with subtype-specific blockers. Overexpression of Ca(V)2.2 did not increase the absolute size of the EPSC even though somatic N-type current was augmented by severalfold. Thus, the strength of neurotransmission is saturated with regard to levels of Ca(2+) channel expression for both N-type and P/Q-type channels. Overexpression of Ca(2+)-impermeable Ca(V)2.2 subunits decreased EPSC size, corroborating competition for channel slots. Striking asymmetries between N- and P/Q-type channels emerged when their relative contributions were compared with channel overexpression. Overexpressed N-type channels could competitively displace P/Q-type channels from P/Q-preferring slots and take over the role of supporting transmission. The converse was not found with overexpression of P/Q-type channels, regardless of their C-terminal domain. We interpret these findings in terms of two different kinds of presynaptic slots at excitatory synapses, one accepting N-type channels but rejecting P/Q-type (N(specific)) and the other preferring P/Q-type but also accepting N-type (PQ(preferring)). The interaction between channels and slots governs the respective contributions of multiple channel types to neurotransmission and, in turn, the ability of transmission to respond to various stimulus patterns and neuromodulators.
Figures




References
-
- Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci. 2007;8:451–465. - PubMed
-
- Butz S, Okamoto M, Südhof TC. A tripartite protein complex with the potential to couple synaptic vesicle exocytosis to cell adhesion in brain. Cell. 1998;94:773–782. - PubMed
-
- Cao YQ, Tsien RW. Differential disposition of N- and P/Q-type Ca2+ channels relative to presynaptic release machinery in cultured hippocampal neurons. Soc Neurosci Abstr. 2006;32:333–6.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
