Role of G-proteins in odor-sensing and CO2-sensing neurons in Drosophila
- PMID: 20357107
- PMCID: PMC2858456
- DOI: 10.1523/JNEUROSCI.6357-09.2010
Role of G-proteins in odor-sensing and CO2-sensing neurons in Drosophila
Abstract
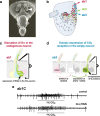
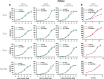
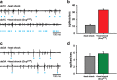
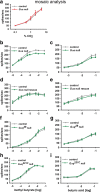
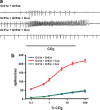
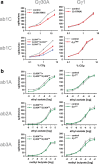
A central question in insect chemoreception is whether signaling occurs via G-proteins. Two families of seven-transmembrane-domain chemoreceptors, the odor (Or) and gustatory receptor (Gr) families, have been identified in Drosophila (Clyne et al., 1999, 2000; Vosshall et al., 1999). Ors mediate odor responses, whereas two Grs, Gr21a and Gr63a, mediate CO2 response (Hallem et al., 2004; Jones et al., 2007; Kwon et al., 2007). Using single-sensillum recordings, we systematically investigate the role of Galpha proteins in vivo, initially with RNA interference constructs, competitive peptides, and constitutively active Galpha proteins. The results do not support a role for Galpha proteins in odor sensitivity. In parallel experiments, manipulations of Galpha(q), but not other Galpha proteins, affected CO2 response. Transient, conditional, and ectopic expression analyses consistently supported a role for Galpha(q) in the response of CO2-sensing neurons, but not odor-sensing neurons. Genetic mosaic analysis confirmed that odor responses are normal in the absence of Galpha(q). Ggamma30A is also required for normal CO2 response. The simplest interpretation of these results is that Galpha(q) and Ggamma30A play a role in the response of CO2-sensing neurons, but are not required for Or-mediated odor signaling.
Figures

References
-
- Akhter SA, Luttrell LM, Rockman HA, Iaccarino G, Lefkowitz RJ, Koch WJ. Targeting the receptor-Gq interface to inhibit in vivo pressure overload myocardial hypertrophy. Science. 1998;280:574–577. - PubMed
-
- Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991;349:117–127. - PubMed
-
- Carlson JR. Olfaction in Drosophila: from odor to behavior. Trends Genet. 1996;12:175–180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
