The angiogenic factor angiopoietin-1 is a proneurogenic peptide on subventricular zone stem/progenitor cells
- PMID: 20357108
- PMCID: PMC6632326
- DOI: 10.1523/JNEUROSCI.5597-09.2010
The angiogenic factor angiopoietin-1 is a proneurogenic peptide on subventricular zone stem/progenitor cells
Abstract
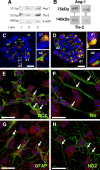
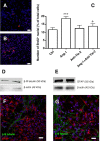
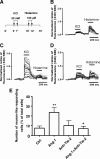
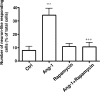
In the adult mammalian brain, the subventricular zone (SVZ) hosts stem cells constantly generating new neurons. Angiopoietin-1 (Ang-1) is an endothelial growth factor with a critical role in division, survival, and adhesion of endothelial cells via Tie-2 receptor activity. Expression of Tie-2 in nonendothelial cells, especially neurons and stem cells, suggests that Ang-1 may be involved in neurogenesis. In the present work, we investigated the putative role of Ang-1 on SVZ neurogenesis. Immature cells from SVZ-derived neurospheres express Ang-1 and Tie-2 mRNA, suggesting a role for the Ang-1/Tie-2 system in the neurogenic niche. Moreover, we also found that Tie-2 protein expression is retained on differentiation in neurons and glial cells. Ang-1 triggered proliferation via activation of the ERK1/2 (extracellular signal-regulated kinase 1/2) mitogen-activated protein kinase (MAPK) kinase pathway but did not induce cell death. Accordingly, coincubation with an anti-Tie-2 neutralizing antibody prevented the pro-proliferative effect of Ang-1. Furthermore, Ang-1 increased the number of NeuN (neuronal nuclear protein)-positive neurons in cultures treated for 7 d, as well as the number of functional neurons, as assessed by monitoring [Ca(2+)](i) rises after application of specific stimuli for neurons and immature cells. The proneurogenic effect of Ang-1 is mediated by Tie-2 activation and subsequent mTOR (mammalian target of rapamycin kinase) mobilization. In agreement, neuronal differentiation significantly decreased after exposure to an anti-Tie-2 neutralizing antibody and to rapamycin. Moreover, Ang-1 elicited the activation of the SAPK (stress-activated protein kinase)/JNK (c-Jun N-terminal kinase) MAPK, involved in axonogenesis. Our work shows a proneurogenic effect of Ang-1, highlighting the relevance of blood vessel/stem cell cross talk in health and disease.
Figures



Similar articles
-
Tumor necrosis factor-alpha modulates survival, proliferation, and neuronal differentiation in neonatal subventricular zone cell cultures.Stem Cells. 2008 Sep;26(9):2361-71. doi: 10.1634/stemcells.2007-0914. Epub 2008 Jun 26. Stem Cells. 2008. PMID: 18583543
-
Activation of type 1 cannabinoid receptor (CB1R) promotes neurogenesis in murine subventricular zone cell cultures.PLoS One. 2013 May 21;8(5):e63529. doi: 10.1371/journal.pone.0063529. Print 2013. PLoS One. 2013. PMID: 23704915 Free PMC article.
-
Neuropeptide Y promotes neurogenesis in murine subventricular zone.Stem Cells. 2008 Jun;26(6):1636-45. doi: 10.1634/stemcells.2008-0056. Epub 2008 Apr 3. Stem Cells. 2008. PMID: 18388302
-
Postnatal development of radial glia and the ventricular zone (VZ): a continuum of the neural stem cell compartment.Cereb Cortex. 2003 Jun;13(6):580-7. doi: 10.1093/cercor/13.6.580. Cereb Cortex. 2003. PMID: 12764031 Review.
-
From progenitors to integrated neurons: role of neurotransmitters in adult olfactory neurogenesis.J Chem Neuroanat. 2011 Dec;42(4):304-16. doi: 10.1016/j.jchemneu.2011.05.006. Epub 2011 May 27. J Chem Neuroanat. 2011. PMID: 21641990 Review.
Cited by
-
Formation of the Mouse Internal Capsule and Cerebral Peduncle: A Pioneering Role for Striatonigral Axons as Revealed in Isl1 Conditional Mutants.J Neurosci. 2022 Apr 20;42(16):3344-3364. doi: 10.1523/JNEUROSCI.2291-21.2022. Epub 2022 Mar 10. J Neurosci. 2022. PMID: 35273083 Free PMC article.
-
The angiopoietin-Tie2 pathway regulates Purkinje cell dendritic morphogenesis in a cell-autonomous manner.Cell Rep. 2021 Aug 17;36(7):109522. doi: 10.1016/j.celrep.2021.109522. Cell Rep. 2021. PMID: 34407407 Free PMC article.
-
Characterization of gene expression changes in human neural stem cells and endothelial cells modeling a neurovascular microenvironment.Brain Res Bull. 2020 May;158:9-19. doi: 10.1016/j.brainresbull.2020.02.008. Epub 2020 Feb 21. Brain Res Bull. 2020. PMID: 32092433 Free PMC article.
-
Delayed Treatment with Green Tea Polyphenol EGCG Promotes Neurogenesis After Ischemic Stroke in Adult Mice.Mol Neurobiol. 2017 Jul;54(5):3652-3664. doi: 10.1007/s12035-016-9924-0. Epub 2016 May 20. Mol Neurobiol. 2017. PMID: 27206430
-
Brain evolution and development: adaptation, allometry and constraint.Proc Biol Sci. 2016 Sep 14;283(1838):20160433. doi: 10.1098/rspb.2016.0433. Proc Biol Sci. 2016. PMID: 27629025 Free PMC article. Review.
References
-
- Abdel-Malak NA, Srikant CB, Kristof AS, Magder SA, Di Battista JA, Hussain SN. Angiopoietin-1 promotes endothelial cell proliferation and migration through AP-1-dependent autocrine production of interleukin-8. Blood. 2008;111:4145–4154. - PubMed
-
- Acker T, Beck H, Plate KH. Cell type specific expression of vascular endothelial growth factor and angiopoietin-1 and -2 suggests an important role of astrocytes in cerebellar vascularization. Mech Dev. 2001;108:45–57. - PubMed
-
- Agasse F, Bernardino L, Silva B, Ferreira R, Grade S, Malva JO. Response to histamine allows the functional identification of neuronal progenitors, neurons, astrocytes, and immature cells in subventricular zone cell cultures. Rejuvenation Res. 2008a;11:187–200. - PubMed
-
- Agasse F, Bernardino L, Kristiansen H, Christiansen SH, Ferreira R, Silva B, Grade S, Woldbye DP, Malva JO. Neuropeptide Y promotes neurogenesis in murine subventricular zone. Stem Cells. 2008b;26:1636–1645. - PubMed
-
- Ambrósio AF, Silva AP, Malva JO, Mesquita JF, Carvalho AP, Carvalho CM. Role of desensitization of AMPA receptors on the neuronal viability and on the [Ca2+]i changes in cultured rat hippocampal neurons. Eur J Neurosci. 2000;12:2021–2031. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
