Dysregulation of L-arginine metabolism and bioavailability associated to free plasma heme
- PMID: 20357184
- PMCID: PMC2904256
- DOI: 10.1152/ajpcell.00405.2009
Dysregulation of L-arginine metabolism and bioavailability associated to free plasma heme
Abstract
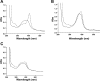
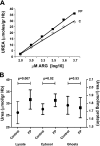
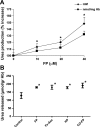
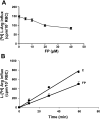
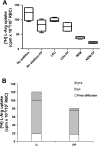
Severe Plasmodium falciparum malaria is associated with hypoargininemia, which contributes to impaired systemic and pulmonary nitric oxide (NO) production and endothelial dysfunction. Since intravascular hemolysis is an intrinsic feature of severe malaria, we investigated whether and by which mechanisms free heme [Fe(III)-protoporphyrin IX (FP)] might contribute to the dysregulation of L-arginine (L-Arg) metabolism and bioavailability. Carrier systems "y+" [or cationic amino acid transporter (CAT)] and "y+L" transport L-Arg into red blood cells (RBC), where it is hydrolyzed to ornithine and urea by arginase (isoform I) or converted to NO* and citrulline by endothelial nitric oxide synthase (eNOS). Our results show a significant and dose-dependent impairment of L-Arg transport into RBC pretreated with FP, with a strong inhibition of the system carrier y+L. Despite the impaired L-Arg influx, higher amounts of L-Arg-derived urea are produced by RBC preexposed to FP caused by activation of RBC arginase I. This activation appeared not to be mediated by oxidative modifications of the enzyme. We conclude that L-Arg transport across RBC membrane is impaired and arginase-mediated L-Arg consumption enhanced by free heme. This could contribute to reduced NO production in severe malaria.
Figures

Similar articles
-
Characterization of cationic amino acid transport systems in rat erythrocytes: lack of effect of uraemia on L-arginine influx.Clin Exp Pharmacol Physiol. 2006 Aug;33(8):702-7. doi: 10.1111/j.1440-1681.2006.04421.x. Clin Exp Pharmacol Physiol. 2006. PMID: 16895543
-
Increased erythrocytes by-products of arginine catabolism are associated with hyperglycemia and could be involved in the pathogenesis of type 2 diabetes mellitus.PLoS One. 2013 Jun 24;8(6):e66823. doi: 10.1371/journal.pone.0066823. Print 2013. PLoS One. 2013. PMID: 23826148 Free PMC article.
-
Downregulation of eNOS and preserved endothelial function in endothelial-specific arginase 1-deficient mice.Nitric Oxide. 2022 Aug 1;125-126:69-77. doi: 10.1016/j.niox.2022.06.004. Epub 2022 Jun 23. Nitric Oxide. 2022. PMID: 35752264
-
Arginine, nitric oxide, carbon monoxide, and endothelial function in severe malaria.Curr Opin Infect Dis. 2008 Oct;21(5):468-75. doi: 10.1097/QCO.0b013e32830ef5cf. Curr Opin Infect Dis. 2008. PMID: 18725795 Free PMC article. Review.
-
Endogenous flux of nitric oxide: Citrulline is preferred to Arginine.Acta Physiol (Oxf). 2021 Mar;231(3):e13572. doi: 10.1111/apha.13572. Epub 2020 Nov 9. Acta Physiol (Oxf). 2021. PMID: 33089645 Review.
Cited by
-
Nitric oxide synthetic pathway in patients with microvascular angina and its relations with oxidative stress.Oxid Med Cell Longev. 2014;2014:726539. doi: 10.1155/2014/726539. Epub 2014 Apr 22. Oxid Med Cell Longev. 2014. PMID: 24864190 Free PMC article.
-
Vascular risk assessment in patients with sickle cell disease.Haematologica. 2011 Jan;96(1):1-5. doi: 10.3324/haematol.2010.035097. Haematologica. 2011. PMID: 21193426 Free PMC article. No abstract available.
-
The blood transcriptome of childhood malaria.EBioMedicine. 2019 Feb;40:614-625. doi: 10.1016/j.ebiom.2018.12.055. Epub 2019 Jan 10. EBioMedicine. 2019. PMID: 30638864 Free PMC article.
-
Decreased Rate of Plasma Arginine Appearance in Murine Malaria May Explain Hypoargininemia in Children With Cerebral Malaria.J Infect Dis. 2016 Dec 15;214(12):1840-1849. doi: 10.1093/infdis/jiw452. J Infect Dis. 2016. PMID: 27923948 Free PMC article.
-
L-Arginine supplementation in mice enhances NO production in spleen cells and inhibits Plasmodium yoelii transmission in mosquitoes.Parasit Vectors. 2015 Jun 14;8:326. doi: 10.1186/s13071-015-0940-0. Parasit Vectors. 2015. PMID: 26070945 Free PMC article.
References
-
- Anstey NM, Weinberg JB, Hassanali MY, Mwaikambo ED, Manyenga D, Misukonis MA, Arnelle DR, Hollis D, McDonald MI, Granger DL. Nitric oxide in Tanzanian children with malaria: inverse relationship between malaria severity and nitric oxide production/nitric oxide synthase type 2 expression. J Exp Med 184: 557–567, 1996 - PMC - PubMed
-
- Bottini E. Association between cytosolic low molecular weight phosphotyrosine-phosphatase and malaria—a possible mechanism. Am J Phys Anthropol 108: 241–244, 1999 - PubMed
-
- Boyd CA, Deves R, Laynes R, Kudo Y, Sebastio G. Cationic amino acid transport through system y+L in erythrocytes of patients with lysinuric protein intolerance. Pflügers Arch 439: 513–516, 2000 - PubMed
-
- Casals-Pascual C, Kai O, Cheung JO, Williams S, Lowe B, Nyanoti M, Williams TN, Maitland K, Molyneux M, Newton CR, Peshu N, Watt SM, Roberts DJ. Suppression of erythropoiesis in malarial anemia is associated with hemozoin in vitro and in vivo. Blood 108: 2569–2577, 2006 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

