Gonadotropins regulate rat testicular tight junctions in vivo
- PMID: 20357222
- PMCID: PMC2875820
- DOI: 10.1210/en.2009-1278
Gonadotropins regulate rat testicular tight junctions in vivo
Abstract
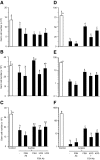
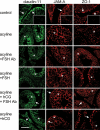
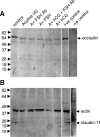
Sertoli cell tight junctions (TJs) are an essential component of the blood-testis barrier required for spermatogenesis; however, the role of gonadotropins in their maintenance is unknown. This study aimed to investigate the effect of gonadotropin suppression and short-term replacement on TJ function and TJ protein (occludin and claudin-11) expression and localization, in an adult rat model in vivo. Rats (n = 10/group) received the GnRH antagonist, acyline, for 7 wk to suppress gonadotropins. Three groups then received for 7 d: 1) human recombinant FSH, 2) human chorionic gonadotropin (hCG) and rat FSH antibody (to study testicular androgen stimulation alone), and 3) hCG alone (to study testicular androgen and pituitary FSH production). TJ proteins were assessed by real-time PCR, Western blot analysis, and immunohistochemistry, whereas TJ function was assessed with a biotin permeation tracer. Acyline treatment significantly reduced testis weights, serum androgens, LH and FSH, and adluminal germ cells (pachytene spermatocyte, round and elongating spermatids). In contrast to controls, acyline induced seminiferous tubule permeability to biotin, loss of tubule lumens, and loss of occludin, but redistribution of claudin-11, immunostaining. Short-term hormone replacement stimulated significant recoveries in adluminal germ cell numbers. In hCG +/- FSH antibody-treated rats, occludin and claudin-11 protein relocalized at the TJ, but such relocalization was minimal with FSH alone. Tubule lumens also reappeared, but most tubules remained permeable to biotin tracer, despite the presence of occludin. It is concluded that gonadotropins maintain Sertoli cell TJs in the adult rat via a mechanism that includes the localization of occludin and claudin-11 at functional TJs.
Figures



Similar articles
-
Gonadotropin suppression in men leads to a reduction in claudin-11 at the Sertoli cell tight junction.Hum Reprod. 2016 Apr;31(4):875-86. doi: 10.1093/humrep/dew009. Epub 2016 Feb 18. Hum Reprod. 2016. PMID: 26908839 Clinical Trial.
-
Claudin-11 expression and localisation is regulated by androgens in rat Sertoli cells in vitro.Reproduction. 2007 Jun;133(6):1169-79. doi: 10.1530/REP-06-0385. Reproduction. 2007. PMID: 17636171
-
Androgen action on the restoration of spermatogenesis in adult rats: effects of human chorionic gonadotrophin, testosterone and flutamide administration on germ cell number.Int J Androl. 1997 Apr;20(2):70-9. doi: 10.1046/j.1365-2605.1997.d01-121.x. Int J Androl. 1997. PMID: 9292316
-
Endocrine control of spermatogenesis in the ram.J Reprod Fertil Suppl. 1981;30:47-60. J Reprod Fertil Suppl. 1981. PMID: 6820054 Review.
-
Endocrinology of male infertility.Br Med Bull. 1979 May;35(2):187-92. doi: 10.1093/oxfordjournals.bmb.a071568. Br Med Bull. 1979. PMID: 387166 Review.
Cited by
-
Structural, cellular and molecular aspects of immune privilege in the testis.Front Immunol. 2012 Jun 11;3:152. doi: 10.3389/fimmu.2012.00152. eCollection 2012. Front Immunol. 2012. PMID: 22701457 Free PMC article.
-
Lentiviral transduction of rat Sertoli cells as a means to modify gene expression.Spermatogenesis. 2012 Oct 1;2(4):279-284. doi: 10.4161/spmg.22516. Spermatogenesis. 2012. PMID: 23248769 Free PMC article.
-
Tetrapeptides Modelled to the Androgen Binding Site of ZIP9 Stimulate Expression of Tight Junction Proteins and Tight Junction Formation in Sertoli Cells.Biology (Basel). 2021 Dec 31;11(1):55. doi: 10.3390/biology11010055. Biology (Basel). 2021. PMID: 35053053 Free PMC article.
-
The Nutraceutical N-Palmitoylethanolamide (PEA) Reveals Widespread Molecular Effects Unmasking New Therapeutic Targets in Murine Varicocele.Nutrients. 2021 Feb 25;13(3):734. doi: 10.3390/nu13030734. Nutrients. 2021. PMID: 33668991 Free PMC article.
-
Organotypic Rat Testicular Organoids for the Study of Testicular Maturation and Toxicology.Front Endocrinol (Lausanne). 2022 Jun 9;13:892342. doi: 10.3389/fendo.2022.892342. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35757431 Free PMC article.
References
-
- Setchell BP 2008 Blood-testis barrier, junctional and transport proteins and spermatogenesis. In: Cheng CY, ed. Molecular mechanisms in spermatogenesis. Austin, TX: Landes Bioscience; 202–233 - PubMed
-
- Russell LD, Peterson RN 1985 Sertoli cell junctions: morphological and functional correlates. Int Rev Cytol 94:177–211 - PubMed
-
- Cavicchia JC, Sacerdote FL, Ortiz L 1996 The human blood-testis barrier in impaired spermatogenesis. Ultrastruct Pathol 20:211–218 - PubMed
-
- Russell LD, Bartke A, Goh JC 1989 Postnatal development of the Sertoli cell barrier, tubular lumen, and cytoskeleton of Sertoli and myoid cells in the rat, and their relationship to tubular fluid secretion and flow. Am J Anat 184:179–189 - PubMed
-
- de Kretser DM, Burger HG 1972 Ultrastructural studies of the human Sertoli cell in normal men and males with hypogonadotropic hypogonadism before and after gonadotropic treatment. In: Saxena BB, Beling CG, Gandy HM, eds. Gonadotropins. New York: Wiley-Interscience; 640–656
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

