Interventions to improve patient comprehension in informed consent for medical and surgical procedures: a systematic review
- PMID: 20357225
- PMCID: PMC5419590
- DOI: 10.1177/0272989X10364247
Interventions to improve patient comprehension in informed consent for medical and surgical procedures: a systematic review
Abstract
Background: Patient understanding in clinical informed consent is often poor. Little is known about the effectiveness of interventions to improve comprehension or the extent to which such interventions address different elements of understanding in informed consent.
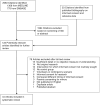
Purpose: . To systematically review communication interventions to improve patient comprehension in informed consent for medical and surgical procedures. Data Sources. A systematic literature search of English-language articles in MEDLINE (1949-2008) and EMBASE (1974-2008) was performed. In addition, a published bibliography of empirical research on informed consent and the reference lists of all eligible studies were reviewed. Study Selection. Randomized controlled trials and controlled trials with nonrandom allocation were included if they compared comprehension in informed consent for a medical or surgical procedure. Only studies that used a quantitative, objective measure of understanding were included. All studies addressed informed consent for a needed or recommended procedure in actual patients. Data Extraction. Reviewers independently extracted data using a standardized form. All results were compared, and disagreements were resolved by consensus. Data Synthesis. Forty-four studies were eligible. Intervention categories included written information, audiovisual/multimedia, extended discussions, and test/feedback techniques. The majority of studies assessed patient understanding of procedural risks; other elements included benefits, alternatives, and general knowledge about the procedure. Only 6 of 44 studies assessed all 4 elements of understanding. Interventions were generally effective in improving patient comprehension, especially regarding risks and general knowledge. Limitations. Many studies failed to include adequate description of the study population, and outcome measures varied widely.
Conclusions: . A wide range of communication interventions improve comprehension in clinical informed consent. Decisions to enhance informed consent should consider the importance of different elements of understanding, beyond procedural risks, as well as feasibility and acceptability of the intervention to clinicians and patients. Conceptual clarity regarding the key elements of informed consent knowledge will help to focus improvements and standardize evaluations.
Figures

References
-
- Whitney SN, McGuire AL, McCullough LB. A typology of shared decision making, informed consent, and simple consent. Ann Intern Med. 2004;140(1):54–9. - PubMed
-
- American Medical Association. Informed consent. [Accessed 27 April 2009]; Available from: URL: http://www.ama-assn.org/ama/pub/physician-resources/legal-topics/patient....
-
- Matiasek J, Wynia MK. Reconceptualizing the informed consent process at eight innovative hospitals. Jt Comm J Qual Patient Saf. 2008;34(3):127–37. - PubMed
-
- Making Healthcare Decisions: The Ethicaland Legal Implications of Informed Consentin the Patient-Practitioner Relationship. Washington, DC: 1982. [Accessed 27 April 2009]. President's Commission for the Study of Ethical Problemsin Medicine and Biomedical and Behavioral Research. Avail-able from: URL: http://www.bioethics.gov/reports/past_commis-sions/index.html.
-
- Resolving Ethical LoB. Dilemmas: A Guide for Clinicians. 2nd. Philadelphia: Lippincott Williams & Wilkins; 2000.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

