CD4+CD25+Foxp3+ regulatory T cells optimize diversity of the conventional T cell repertoire during reconstitution from lymphopenia
- PMID: 20357265
- PMCID: PMC3813316
- DOI: 10.4049/jimmunol.0904076
CD4+CD25+Foxp3+ regulatory T cells optimize diversity of the conventional T cell repertoire during reconstitution from lymphopenia
Abstract
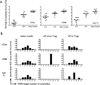
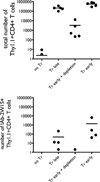
The functional capacity of the adaptive immune system is dependent on the size and the diversity of the T cell population. In states of lymphopenia, T cells are driven to proliferate to restore the T cell population size. However, different T cell clones proliferate at different rates, and some T cells experience burst-like expansion called spontaneous lymphopenia-induced proliferation (LIP). These T cells are likely receiving stimulation from cognate Ags and are most responsible for inflammatory pathology that can emerge in lymphopenic states. Foxp3(+) regulatory T cells (Tregs) selectively inhibit spontaneous LIP, which may contribute to their ability to prevent lymphopenia-associated autoimmunity. We hypothesized that another potential negative consequence of unrestrained spontaneous LIP is constriction of the total T cell repertoire. We demonstrate that the absence of Foxp3(+) Tregs during the period of immune reconstitution results in the development of TCR repertoire "holes" and the loss of Ag-specific responsiveness to infectious microorganisms. In contrast, the presence of Tregs during the period of immune reconstitution preserves optimal TCR diversity and foreign Ag responsiveness. This finding contrasts with the generally accepted immunosuppressive role of Tregs and provides another example of Treg activity that actually enhances immune function.
Figures








Similar articles
-
Cyclophosphamide-induced type-1 diabetes in the NOD mouse is associated with a reduction of CD4+CD25+Foxp3+ regulatory T cells.J Immunol. 2006 Nov 15;177(10):6603-12. doi: 10.4049/jimmunol.177.10.6603. J Immunol. 2006. PMID: 17082572
-
Regulatory CD4+CD25+Foxp3+ T cells selectively inhibit the spontaneous form of lymphopenia-induced proliferation of naive T cells.J Immunol. 2008 Jun 1;180(11):7305-17. doi: 10.4049/jimmunol.180.11.7305. J Immunol. 2008. PMID: 18490730
-
CD4+VEGFR1(HIGH) T cell as a novel Treg subset regulates inflammatory bowel disease in lymphopenic mice.Cell Mol Immunol. 2015 Sep;12(5):592-603. doi: 10.1038/cmi.2015.71. Epub 2015 Jul 27. Cell Mol Immunol. 2015. PMID: 26211666 Free PMC article.
-
Interleukin-7 and immune reconstitution in cancer patients: a new paradigm for dramatically increasing overall survival.Target Oncol. 2012 Mar;7(1):55-68. doi: 10.1007/s11523-012-0210-4. Epub 2012 Mar 2. Target Oncol. 2012. PMID: 22383042 Free PMC article. Review.
-
A causal link between lymphopenia and autoimmunity.Immunol Lett. 2005 Apr 15;98(1):23-31. doi: 10.1016/j.imlet.2004.10.022. Epub 2004 Nov 24. Immunol Lett. 2005. PMID: 15790505 Free PMC article. Review.
Cited by
-
Context and location dependence of adaptive Foxp3(+) regulatory T cell formation during immunopathological conditions.Cell Immunol. 2012 Sep;279(1):60-5. doi: 10.1016/j.cellimm.2012.09.009. Epub 2012 Oct 1. Cell Immunol. 2012. PMID: 23089195 Free PMC article. Review.
-
Lymphopenia-induced lymphoproliferation drives activation of naive T cells and expansion of regulatory populations.iScience. 2021 Feb 7;24(3):102164. doi: 10.1016/j.isci.2021.102164. eCollection 2021 Mar 19. iScience. 2021. PMID: 33665580 Free PMC article.
-
PD-1 Controls Tonic Signaling and Lymphopenia-Induced Proliferation of T Lymphocytes.Front Immunol. 2017 Oct 12;8:1289. doi: 10.3389/fimmu.2017.01289. eCollection 2017. Front Immunol. 2017. PMID: 29075267 Free PMC article.
-
Prior to Peripheral Tolerance, Newly Generated CD4 T Cells Maintain Dangerous Autoimmune Potential: Fas- and Perforin-Independent Autoimmunity Controlled by Programmed Death-1.Front Immunol. 2018 Jan 24;9:12. doi: 10.3389/fimmu.2018.00012. eCollection 2018. Front Immunol. 2018. PMID: 29416537 Free PMC article.
-
Increased circulating CD4+FOXP3+ T cells associate with early relapse following autologous hematopoietic stem cell transplantation in multiple myeloma patients.Oncotarget. 2018 Jun 5;9(43):27305-27317. doi: 10.18632/oncotarget.25553. eCollection 2018 Jun 5. Oncotarget. 2018. PMID: 29930767 Free PMC article.
References
-
- Jameson SC. T cell homeostasis: keeping useful T cells alive and live T cells useful. Semin Immunol. 2005;17:231–237. - PubMed
-
- Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. - PubMed
-
- Min B, McHugh R, Sempowski GD, Mackall C, Foucras G, Paul WE. Neonates support lymphopenia-induced proliferation. Immunity. 2003;18:131–140. - PubMed
-
- Messaoudi I, Warner J, Nikolich-Zugich D, Fischer M, Nikolich-Zugich J. Molecular, Cellular, and Antigen Requirements for Development of Age-Associated T Cell Clonal Expansions In Vivo. J Immunol. 2006;176:301–308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

