Acute and chronic effects of oral genistein administration in neonatal mice
- PMID: 20357267
- PMCID: PMC2888966
- DOI: 10.1095/biolreprod.109.080549
Acute and chronic effects of oral genistein administration in neonatal mice
Abstract
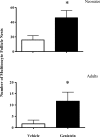
Soy-based infant formulas are widely used in the United States and some other countries. These formulas contain high levels of the estrogenic isoflavone genistein, leading to concern that neonatal genistein exposure could cause acute and/or long-term adverse effects on reproductive and other organs. However, previous work to assess genistein effects in rodent models has not typically replicated the route of delivery and/or serum genistein concentrations reported for soy formula-fed human infants. Our objective was to develop a mouse model that more closely mimics the oral genistein exposure and total serum genistein concentrations observed in soy formula-fed infants. Mouse pups were dosed orally with genistein in a soy formula-corn oil emulsion from Postnatal Day (PND) 1 to PND 5, then effects on reproductive and non-reproductive organs were assessed after dosing and during subsequent development. Neonatal treatment resulted in changes both at the completion of dosing (PND 5) and in adult animals. At PND 5, neonatal genistein treatment caused increased relative uterine weight and down-regulation of progesterone receptor in uterine epithelia. Estrogenic effects of genistein were also seen in the neonatal ovary and thymus, which had an increase in the incidence of multioocyte follicles (MOFs) and a decrease in thymic weight relative to body weight, respectively. The increased incidence of MOFs persisted into adulthood for neonatally treated genistein females, and estrous cycle abnormalities were seen at 6 mo of age despite normal fertility in these mice. The immediate and long-term effects in this neonatal animal model raise concerns that high serum concentrations of genistein are estrogenic and could potentially impact the development of human infants fed soy formula.
Figures

Similar articles
-
Multigenerational reproductive study of genistein (Cas No. 446-72-0) in Sprague-Dawley rats (feed study).Natl Toxicol Program Tech Rep Ser. 2008 Mar;(539):1-266. Natl Toxicol Program Tech Rep Ser. 2008. PMID: 18685713
-
NTP-CERHR expert panel report on the developmental toxicity of soy infant formula.Birth Defects Res B Dev Reprod Toxicol. 2011 Oct;92(5):421-68. doi: 10.1002/bdrb.20314. Epub 2011 Sep 21. Birth Defects Res B Dev Reprod Toxicol. 2011. PMID: 21948615
-
NTP toxicity report of reproductive dose range-finding study of Genistein (CAS No. 446-72-0) administered in feed to Sprague-Dawley rats.Toxic Rep Ser. 2007 Nov;(79):1-C2. Toxic Rep Ser. 2007. PMID: 18685712
-
Disruption of the developing female reproductive system by phytoestrogens: genistein as an example.Mol Nutr Food Res. 2007 Jul;51(7):832-44. doi: 10.1002/mnfr.200600258. Mol Nutr Food Res. 2007. PMID: 17604387 Review.
-
Disruption of the female reproductive system by the phytoestrogen genistein.Reprod Toxicol. 2007 Apr-May;23(3):308-16. doi: 10.1016/j.reprotox.2006.11.012. Epub 2006 Dec 9. Reprod Toxicol. 2007. PMID: 17250991 Review.
Cited by
-
Diverse effects of phytoestrogens on the reproductive performance: cow as a model.Int J Endocrinol. 2013;2013:650984. doi: 10.1155/2013/650984. Epub 2013 Apr 23. Int J Endocrinol. 2013. PMID: 23710176 Free PMC article.
-
Genistein inhibits proliferation and induces senescence in neonatal mouse pituitary gland explant cultures.Toxicology. 2019 Nov 1;427:152306. doi: 10.1016/j.tox.2019.152306. Epub 2019 Oct 5. Toxicology. 2019. PMID: 31593742 Free PMC article.
-
In vivo inhibition of BCRP/ABCG2 mediated transport of nitrofurantoin by the isoflavones genistein and daidzein: a comparative study in Bcrp1 (-/-) mice.Pharm Res. 2010 Oct;27(10):2098-105. doi: 10.1007/s11095-010-0208-5. Epub 2010 Jul 7. Pharm Res. 2010. PMID: 20607366
-
Phytobioactive compound-based nanodelivery systems for the treatment of type 2 diabetes mellitus - current status.Int J Nanomedicine. 2017 Feb 9;12:1097-1111. doi: 10.2147/IJN.S124601. eCollection 2017. Int J Nanomedicine. 2017. PMID: 28223801 Free PMC article. Review.
-
Coexposure to phytoestrogens and bisphenol a mimics estrogenic effects in an additive manner.Toxicol Sci. 2014 Mar;138(1):21-35. doi: 10.1093/toxsci/kft271. Epub 2013 Nov 27. Toxicol Sci. 2014. PMID: 24284790 Free PMC article.
References
-
- Bhatia J, Greer F.Use of soy protein-based formulas in infant feeding. Pediatrics 2008; 121: 1062–1068. - PubMed
-
- Strom BL, Schinnar R, Ziegler EE, Barnhart KT, Sammel MD, Macones GA, Stallings VA, Drulis JM, Nelson SE, Hanson SA.Exposure to soy-based formula in infancy and endocrinological and reproductive outcomes in young adulthood. JAMA 2001; 286: 807–814. - PubMed
-
- Berger-Achituv S, Shohat T, Romano-Zelekha O, Ophir E, Rachmani S, Malovizky D, Garty BZ.Widespread use of soy-based formula without clinical indications. J Pediatr Gastroenterol Nutr 2005; 41: 660–666. - PubMed
-
- Agostoni C, Axelsson I, Goulet O, Koletzko B, Michaelsen KF, Puntis J, Rieu D, Rigo J, Shamir R, Szajewska H, Turck D.Soy protein infant formulae and follow-on formulae: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr 2006; 42: 352–361. - PubMed
-
- Badger TM, Gilchrist JM, Pivik RT, Andres A, Shankar K, Chen JR, Ronis MJ.The health implications of soy infant formula. Am J Clin Nutr 2009; 89: 1668S–1672S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

