First evidence of bone morphogenetic protein 1 expression and activity in sheep ovarian follicles
- PMID: 20357269
- PMCID: PMC2888967
- DOI: 10.1095/biolreprod.109.082115
First evidence of bone morphogenetic protein 1 expression and activity in sheep ovarian follicles
Abstract
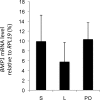
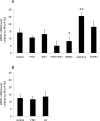
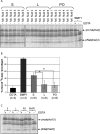
Bone morphogenetic protein (BMP) 1 is a vertebrate metalloproteinase of the astacin family. BMP1 plays a key role in regulating the formation of the extracellular matrix (ECM), particularly by processing the C-propeptide of fibrillar procollagens. BMP1 also promotes BMP signaling by releasing BMP signaling molecules from complexes with the BMP-antagonist chordin. As a result of BMP1's dual role in both ECM formation and BMP signaling, we hypothesized that BMP1 could play a role in ovarian physiology. Using the sheep ovary as a model system, we showed that BMP1 was expressed in the ovary throughout early fetal stages to adulthood. Furthermore, in adult ovaries, BMP1 was expressed along with chordin, BMP4, and twisted gastrulation, which together form an extracellular regulatory complex for BMP signaling. Within ovine ovaries, immunohistochemical localization demonstrated that BMP1 was present in granulosa cells at all stages of follicular development, from primordial to large antral follicles, and that the levels of BMP1 were not affected by the final follicle selection mechanism. In cultured granulosa cells, BMP1 expression was not affected by gonadotropins, but BMP4 and activin A had opposing effects on the levels of BMP1 mRNA. BMP1 appeared to be secreted into the follicular fluid of antral follicles, where it is able to exert procollagen C-proteinase and chordinase activities. Interestingly, BMP1 activity in follicular fluid decreased with follicular growth.
Figures




Similar articles
-
Developmental roles of the BMP1/TLD metalloproteinases.Birth Defects Res C Embryo Today. 2006 Mar;78(1):47-68. doi: 10.1002/bdrc.20060. Birth Defects Res C Embryo Today. 2006. PMID: 16622848 Review.
-
Use of Bmp1/Tll1 doubly homozygous null mice and proteomics to identify and validate in vivo substrates of bone morphogenetic protein 1/tolloid-like metalloproteinases.Mol Cell Biol. 2003 Jul;23(13):4428-38. doi: 10.1128/MCB.23.13.4428-4438.2003. Mol Cell Biol. 2003. PMID: 12808086 Free PMC article.
-
BMP-1 participates in the selection and dominance of buffalo follicles by regulating the proliferation and apoptosis of granulosa cells.Theriogenology. 2016 Mar 15;85(5):999-1012. doi: 10.1016/j.theriogenology.2015.11.011. Epub 2015 Nov 25. Theriogenology. 2016. PMID: 26778140
-
Fibronectin binds and enhances the activity of bone morphogenetic protein 1.J Biol Chem. 2009 Sep 18;284(38):25879-88. doi: 10.1074/jbc.M109.024125. Epub 2009 Jul 18. J Biol Chem. 2009. PMID: 19617627 Free PMC article.
-
Development of the dominant follicle: mechanisms of selection and maintenance of oocyte quality.Soc Reprod Fertil Suppl. 2007;64:141-63. doi: 10.5661/rdr-vi-141. Soc Reprod Fertil Suppl. 2007. PMID: 17491145 Review.
Cited by
-
The expression and mutation of BMPR1B and its association with litter size in small-tail Han sheep (Ovis aries).Arch Anim Breed. 2021 May 28;64(1):211-221. doi: 10.5194/aab-64-211-2021. eCollection 2021. Arch Anim Breed. 2021. PMID: 34109270 Free PMC article.
-
An overview of gene expression dynamics during early ovarian folliculogenesis: specificity of follicular compartments and bi-directional dialog.BMC Genomics. 2013 Dec 19;14:904. doi: 10.1186/1471-2164-14-904. BMC Genomics. 2013. PMID: 24350644 Free PMC article.
-
Bone morphogenetic protein 1 is expressed in porcine ovarian follicles and promotes oocyte maturation and early embryonic development.J Vet Med Sci. 2017 Feb 4;79(2):258-266. doi: 10.1292/jvms.16-0277. Epub 2016 Nov 25. J Vet Med Sci. 2017. PMID: 27890905 Free PMC article.
-
A unique 15-bp InDel in the first intron of BMPR1B regulates its expression in Taihu pigs.BMC Genomics. 2022 Dec 3;23(1):799. doi: 10.1186/s12864-022-08988-6. BMC Genomics. 2022. PMID: 36463109 Free PMC article.
-
Multifaceted Functions of TWSG1: From Embryogenesis to Cancer Development.Int J Mol Sci. 2022 Oct 22;23(21):12755. doi: 10.3390/ijms232112755. Int J Mol Sci. 2022. PMID: 36361543 Free PMC article. Review.
References
-
- Wozney J, Rosen V, Celeste A, Mitsock L, Whitters M, Kriz R, Hewick R, Wang E.Novel regulators of bone formation: molecular clones and activities. Science 1988; 242: 1528–1534. - PubMed
-
- Takahara K, Lyons GE, Greenspan DS.Bone morphogenetic protein-1 and a mammalian tolloid homologue (mTld) are encoded by alternatively spliced transcripts which are differentially expressed in some tissues. J Biol Chem 1994; 269: 32572–32578. - PubMed
-
- Dumermuth E, Sterchi E, Jiang W, Wolz R, Bond J, Flannery A, Beynon R.The astacin family of metalloendopeptidases. J Biol Chem 1991; 266: 21381–21385. - PubMed
-
- Davis CG.The many faces of epidermal growth factor repeats. New Biol 1990; 2: 410–419. - PubMed
-
- Handford PA, Mayhew M, Baron M, Winship PR, Campbell ID, Brownlee GG.Key residues involved in calcium-binding motifs in EGF-like domains. Nature 1991; 351: 164–167. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

