Simultaneous detection of circulating autoreactive CD8+ T-cells specific for different islet cell-associated epitopes using combinatorial MHC multimers
- PMID: 20357361
- PMCID: PMC2889772
- DOI: 10.2337/db09-1486
Simultaneous detection of circulating autoreactive CD8+ T-cells specific for different islet cell-associated epitopes using combinatorial MHC multimers
Abstract
Objective: Type 1 diabetes results from selective T-cell-mediated destruction of the insulin-producing beta-cells in the pancreas. In this process, islet epitope-specific CD8(+) T-cells play a pivotal role. Thus, monitoring of multiple islet-specific CD8(+) T-cells may prove to be valuable for measuring disease activity, progression, and intervention. Yet, conventional detection techniques (ELISPOT and HLA tetramers) require many cells and are relatively insensitive.
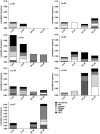
Research design and methods: Here, we used a combinatorial quantum dot major histocompatibility complex multimer technique to simultaneously monitor the presence of HLA-A2 restricted insulin B(10-18), prepro-insulin (PPI)(15-24), islet antigen (IA)-2(797-805), GAD65(114-123), islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP)(265-273), and prepro islet amyloid polypeptide (ppIAPP)(5-13)-specific CD8(+) T-cells in recent-onset diabetic patients, their siblings, healthy control subjects, and islet cell transplantation recipients.
Results: Using this kit, islet autoreactive CD8(+) T-cells recognizing insulin B(10-18), IA-2(797-805), and IGRP(265-273) were shown to be frequently detectable in recent-onset diabetic patients but rarely in healthy control subjects; PPI(15-24) proved to be the most sensitive epitope. Applying the "Diab-Q-kit" to samples of islet cell transplantation recipients allowed detection of changes of autoreactive T-cell frequencies against multiple islet cell-derived epitopes that were associated with disease activity and correlated with clinical outcome.
Conclusions: A kit was developed that allows simultaneous detection of CD8(+) T-cells reactive to multiple HLA-A2-restricted beta-cell epitopes requiring limited amounts of blood, without a need for in vitro culture, that is applicable on stored blood samples.
Figures



References
-
- In't Veld P, Lievens D, De Grijse J, Ling Z, Van der Auwera B, Pipeleers-Marichal M, Gorus F, Pipeleers D: Screening for insulitis in adult autoantibody-positive organ donors. Diabetes 2007;56:2400–2404 - PubMed
-
- Bottazzo GF, Dean BM, McNally JM, MacKay EH, Swift PG, Gamble DR: In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N Engl J Med 1985;313:353–360 - PubMed
-
- Foulis AK, Liddle CN, Farquharson MA, Richmond JA, Weir RS: The histopathology of the pancreas in type 1 (insulin-dependent) diabetes mellitus: a 25-year review of deaths in patients under 20 years of age in the United Kingdom. Diabetologia 1986;29:267–274 - PubMed
-
- Santamaria P, Utsugi T, Park BJ, Averill N, Kawazu S, Yoon JW: Beta-cell-cytotoxic CD8+ T cells from nonobese diabetic mice use highly homologous T cell receptor alpha-chain CDR3 sequences. J Immunol 1995;154:2494–2503 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

