Further improvement in postprandial glucose control with addition of exenatide or sitagliptin to combination therapy with insulin glargine and metformin: a proof-of-concept study
- PMID: 20357372
- PMCID: PMC2890351
- DOI: 10.2337/dc09-2191
Further improvement in postprandial glucose control with addition of exenatide or sitagliptin to combination therapy with insulin glargine and metformin: a proof-of-concept study
Abstract
Objective: To assess the effect of a 4-week adjunctive therapy of exenatide (EXE) (5-10 microg b.i.d.) or sitagliptin (SITA) (100 mg once daily) in response to a standardized breakfast meal challenge in 48 men or women with type 2 diabetes receiving insulin glargine (GLAR) + metformin (MET).
Research design and methods: This was a single-center, randomized, open-label, active comparator-controlled study with a three-arm parallel group design, consisting of: screening, 4- to 8-week run-in period, 4-week treatment period, and follow-up. In all three groups, the GLAR dose was titrated according to an algorithm (fasting blood glucose <or=100 mg/dl).
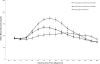
Results: The unadjusted 6-h postprandial blood glucose excursion of both GLAR + MET + EXE and GLAR + MET + SITA was statistically significantly smaller than that of GLAR + MET (606 +/- 104 vs. 612 +/- 133 vs. 728 +/- 132 mg/dl/h; P = 0.0036 and 0.0008). A1C significantly decreased in all three groups (P < 0.0001), with the greatest reduction of -1.9 +/- 0.7 under GLAR + MET + EXE (GLAR + MET + SITA -1.5 +/- 0.7; GLAR + MET -1.2 +/- 0.5%-points; GLAR + MET + EXE vs. GLAR + MET P = 0.0154). The American Diabetes Association A1C target of <7.0% was reached by 80.0, 87.5, and 62.5% of subjects, respectively. GLAR + MET + EXE had the highest number (47) of adverse events, mostly gastrointestinal (56%) with one dropout. GLAR + MET or GLAR + MET + SITA only had 10 and 12 adverse events, respectively, and no dropouts. Hypoglycemia (blood glucose <50 mg/dl) rates were low and comparable among groups. Weight decreased with GLAR + MET + EXE (-0.9 +/- 1.7 kg; P = 0.0396) and increased slightly with GLAR + MET (0.4 +/- 1.5 kg; NS; GLAR + MET + EXE vs. GLAR + MET P = 0.0377).
Conclusions: EXE or SITA added to GLAR + MET further substantially reduced postprandial blood glucose excursions. Longer-term studies in a larger population are warranted to confirm these findings.
Trial registration: ClinicalTrials.gov NCT00971659.
Figures
Similar articles
-
Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial.Ann Intern Med. 2005 Oct 18;143(8):559-69. doi: 10.7326/0003-4819-143-8-200510180-00006. Ann Intern Med. 2005. PMID: 16230722 Clinical Trial.
-
Glucagon-like peptide 1 receptor agonist or bolus insulin with optimized basal insulin in type 2 diabetes.Diabetes Care. 2014 Oct;37(10):2763-73. doi: 10.2337/dc14-0876. Epub 2014 Jul 10. Diabetes Care. 2014. PMID: 25011946 Clinical Trial.
-
Effects of exenatide on measures of β-cell function after 3 years in metformin-treated patients with type 2 diabetes.Diabetes Care. 2011 Sep;34(9):2041-7. doi: 10.2337/dc11-0291. Diabetes Care. 2011. PMID: 21868779 Free PMC article. Clinical Trial.
-
Encapsulation of exenatide in poly-(D,L-lactide-co-glycolide) microspheres produced an investigational long-acting once-weekly formulation for type 2 diabetes.Diabetes Technol Ther. 2011 Nov;13(11):1145-54. doi: 10.1089/dia.2011.0050. Epub 2011 Jul 13. Diabetes Technol Ther. 2011. PMID: 21751887 Free PMC article. Review.
-
Diabetes and cancer: 5 years into the recent controversy.Eur J Cancer. 2014 Aug;50(12):2119-25. doi: 10.1016/j.ejca.2014.04.032. Epub 2014 Jun 11. Eur J Cancer. 2014. PMID: 24930060 Review.
Cited by
-
Emerging role of insulin with incretin therapies for management of type 2 diabetes.Diabetes Ther. 2011 Sep;2(3):146-61. doi: 10.1007/s13300-011-0005-0. Epub 2011 Jul 21. Diabetes Ther. 2011. PMID: 22127824 Free PMC article.
-
GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus.Nat Rev Endocrinol. 2012 Dec;8(12):728-42. doi: 10.1038/nrendo.2012.140. Epub 2012 Sep 4. Nat Rev Endocrinol. 2012. PMID: 22945360 Review.
-
Effects of exenatide twice daily versus sitagliptin on 24-h glucose, glucoregulatory and hormonal measures: a randomized, double-blind, crossover study.Diabetes Obes Metab. 2011 Nov;13(11):982-9. doi: 10.1111/j.1463-1326.2011.01428.x. Diabetes Obes Metab. 2011. PMID: 21615670 Free PMC article. Clinical Trial.
-
Comparative effectiveness of dipeptidylpeptidase-4 inhibitors in type 2 diabetes: a systematic review and mixed treatment comparison.Diabetes Ther. 2014 Jun;5(1):1-41. doi: 10.1007/s13300-014-0061-3. Epub 2014 Mar 25. Diabetes Ther. 2014. PMID: 24664619 Free PMC article.
-
Combining basal insulin analogs with glucagon-like peptide-1 mimetics.Diabetes Technol Ther. 2011 Sep;13(9):873-81. doi: 10.1089/dia.2010.0250. Epub 2011 Jun 28. Diabetes Technol Ther. 2011. PMID: 21711120 Free PMC article. Review.
References
-
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352: 837–853 - PubMed
-
- Nathan DM, Buse JB, Davidson MB, Heine RJ, Holman RR, Sherwin R, Zinman B: Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2006; 29: 1963–1972 - PubMed
-
- van Avendonk MJ, Rutten GE: Insulin therapy in type 2 diabetes: what is the evidence? Diabetes Obes Metab 2009; 11: 415–432 - PubMed
-
- Mäkimattila S, Nikkilä K, Yki-Järvinen H: Causes of weight gain during insulin therapy with and without metformin in patients with type II diabetes mellitus. Diabetologia 1999; 42: 406–412 - PubMed
-
- Klein S, Sheard NF, Pi-Sunyer X, Daly A, Wylie-Rosett J, Kulkarni K, Clark NG: Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies: a statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Diabetes Care 2004; 27: 2067–2073 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous