Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: case report of an unforeseen adverse event in a phase I clinical trial
- PMID: 20357779
- PMCID: PMC2862525
- DOI: 10.1038/mt.2010.31
Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: case report of an unforeseen adverse event in a phase I clinical trial
Figures
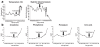
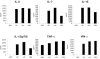
Comment in
-
Safer CARS.Mol Ther. 2010 Apr;18(4):661-2. doi: 10.1038/mt.2010.42. Mol Ther. 2010. PMID: 20357776 Free PMC article. No abstract available.
References
-
- Sadelain M, Rivière I., and , Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 2003;3:35–45. - PubMed
-
- Brentjens RJ, Latouche JB, Santos E, Marti F, Gong MC, Lyddane C.et al.(2003Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15 Nat Med 9279–286. - PubMed
-
- Maher J, Brentjens R, Gunset G, Rivière I., and , Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat Biotechnol. 2002;20:70–75. - PubMed
-
- Brentjens RJ, Santos E, Nikhamin Y, Yeh R, Matsushita M, La Perle K.et al. (2007Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts Clin Cancer Res 135426–5435. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

