Carbohydrate vaccines: developing sweet solutions to sticky situations?
- PMID: 20357803
- PMCID: PMC3878310
- DOI: 10.1038/nrd3012
Carbohydrate vaccines: developing sweet solutions to sticky situations?
Abstract
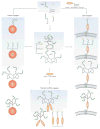
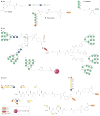
Recent technological advances in glycobiology and glycochemistry are paving the way for a new era in carbohydrate vaccine design. This is enabling greater efficiency in the identification, synthesis and evaluation of unique glycan epitopes found on a plethora of pathogens and malignant cells. Here, we review the progress being made in addressing challenges posed by targeting the surface carbohydrates of bacteria, protozoa, helminths, viruses, fungi and cancer cells for vaccine purposes.
Figures

Comment in
-
Pneumococcal glycoconjugate vaccines produce antibody responses that strongly correlate with function.Nat Rev Drug Discov. 2011 May;10(5):393. doi: 10.1038/nrd3012-c1. Nat Rev Drug Discov. 2011. PMID: 21532568 No abstract available.
References
-
- Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis. 2008;47:401–9. - PubMed
-
- Heidelberger M, Dilapi MM, Siegel M, Walter AW. Persistence of Antibodies in Human Subjects Injected with Pneumococcal Polysaccharides. Journal of Immunology. 1950;65:535–541. - PubMed
-
- Merck & Co., Inc. Pneumovax 23 (pneumococcal vaccine polyvalent) Whitehouse Station, NJ: Merck; 2009. website [online]
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

